Spot Capture and Detection Peptide Tag and Nanobody
The Spot Nanobody for multiple capture & detection applications of Spot-tagged proteins
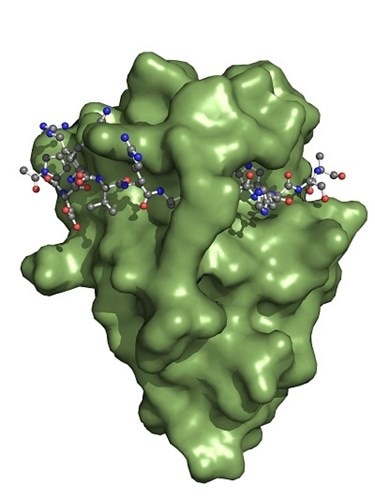
The Spot-Nanobody (green) binds to the Spot-Tag sequence motif PDRVRAVSHWSS. Upon binding, the Spot-Tag peptide is embedded on the surface of the Spot-Nanobody and becomes a β-sheet extension of the Spot-VHH. Defined interactions of the Spot-Nanobody’s side chains to the Spot-peptide determine specificity. In addition, the Spot-peptide is clamped by two amino acid side chains of the Spot-Nanobody. This binding mechanism elucidates why the Spot-Nanobody binds with high affinity to the Spot-Tag.
Content:
Very low background capture and detection systems
Introduction of the Spot Peptide-Tag & Spot-Nanobody
for Capture and Detection Applications
ChromoTek’s innovative Spot-System is the first Nanobody (or VHH, single domain antibody) for multiple capture & detection applications of Spot-peptide-tagged proteins. It comprises Spot-Tag®, an inert 12 amino acid peptide-tag: PDRVRAVSHWSS, which has been affinity optimized, and the small size Spot-Nanobody that specifically binds to Spot-tagged proteins with high affinity and low background.
ChromoTek Spot-Nanobody is preconjugated and ready to use as a Nano-Trap and a Nano-Label, but is also offered as uncoupled VHH:
- For immunoprecipitation, the Spot-Nanobody is coupled to beads and called Spot-Trap®.
- For protein purification, an engineered Spot-Nanobody is coupled to agarose: Spot-Cap®.
- For immunofluorescence and Western blotting, the Spot-Nanobody is conjugated to fluorescent dyes and called Spot-Label®.
- For immobilization and coupling via NHS Ester reaction, the Spot-Nanobody is called Spot VHH, recombinant binding protein.
Origin of Spot-Tag
The Spot-Tag® has been engineered from a linear epitope of the unstructured N-terminus of beta-catenin. Its wild-type sequence has been affinity optimized: The Spot-Tag peptide is bound with a high affinity of 0.7 nM. Hence, the Spot-Nanobody binds better to Spot-tagged proteins than to wildtype beta-catenin not only when the Spot-tagged protein is present at heterologous expression levels, but also at endogenous expression levels when the Spot-tag has been introduced by e.g. CRISPR/Cas to the endogenous gene of the protein of interest.
See Application Note Spot-Trap and Spot-Label for CRISPR’ed Spot-Tag
The Spot-Nanobody and Tag is a very low background capture and detection system
Spot-Tag is a very low background peptide tag
Experimental data from immunoprecipitation and immunofluorescence experiments show that the beta-catenin background, i.e. binding of beta-catenin to the Spot-Nanobody is negligible. However, for very sensitive methods such as Mass Spectrometry, binding to beta-catenin-binding may be still detectable, as well as to some of the beta-catenin interaction partners such as alpha-catenin. Since these are known contaminants, these peptides can be easily subtracted during data analysis or used for alignments. Additionally, our data indicate that Spot-Trap only binds to beta-catenin, if this epitope is dephosphorylated, which reduces background levels even further.
Spot-Label is a very low background detection reagent
For immunofluorescence applications, Virant et al 2018 in Nature Communications, doi:10.1038/s41467-018-03191-2, have carefully determined that the Spot-Nanobody’s background signals from binding to endogenous beta-catenin have only a minor impact on immunostaining. Note that the Spot-Nanobody is called BC2-Nanobody in this publication. Also, our own imaging data shows no background from endogenous beta-catenin.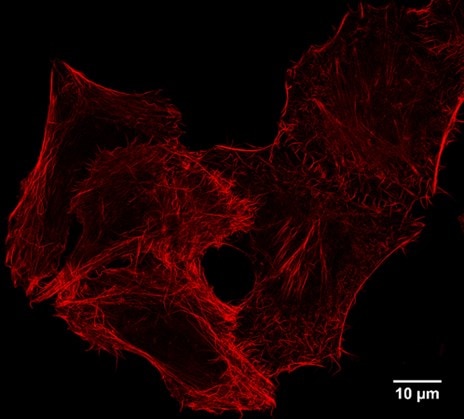
STED super resolution imaging: IF of Spot-tagged Actin-Chromobody with Spot-Label Atto594 bivalent (1:1,000). Gated STED images were acquired with a Leica TCS SP8 STED 3X microscope with pulsed White Light Laser excitation at 590 nm and pulsed depletion with a 775 nm laser. Objective: 100x Oil STED White, NA: 1.4. Pixel size: 21 x 21 nm; z-Step size of z-Stacks: 0.16 µm. Images were deconvolved with Huygens Professional (SVI). STED images were recorded at the Core Facility Bioimaging at the Biomedical Center, LMU Munich.
Spot-Trap is a very low background capture reagent
For immunoprecipitation (IP) applications, Spot-Trap has been benchmarked with commonly used affinity reagents from other suppliers. IPs were done using HEK293T cell lysates without Spot-Tag present, normalized for equal binding capacity, and carried out according to the suppliers’ protocols: This comparison has shown that Spot-Trap has the lowest background of all tested products.
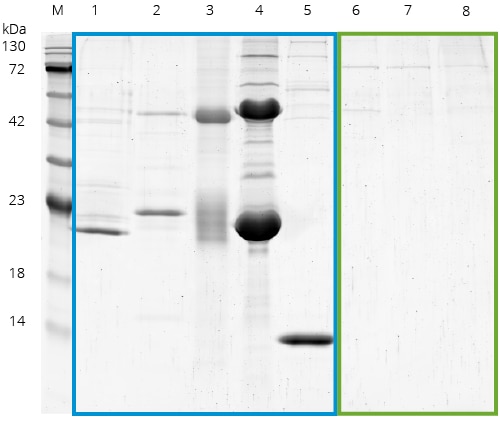
Benchmarking of common affinity resins including Spot: SDS-PAGE of a benchmark of Spot with commonly used affinity reagents from other suppliers. IPs were done using HEK293T cell lysates without Spot-Tag or any other tag present, normalized for equal binding capacity, and carried out according to the suppliers’ protocols.
M: Marker, 1: GST beads, 2: anti-Myc 9E10 antibody beads (supplier A), 3: anti-Myc 9E10 antibody beads (supplier B), 4: anti-Flag beads, 5: Streptavidin conjugated Magnetic Particles M-270, 6: Spot-Trap Agarose, 7: Spot-Trap Magnetic Agarose, 8: Spot-Trap Magnetic Particles M-270. Lanes 1-5 (blue box) show the unspecific binding of cellular proteins. In contrast, the Spot-Traps (lanes 6-8, green box)
have significantly less background.: Note that Spot-Trap has the lowest background of all tested products.
Applications overview
Protein purification
Spot-tagged proteins may be purified using Spot-Cap (i.e. engineered anti-Spot-Nanobody covalently coupled to agarose beads). Bound Spot-tagged proteins can be eluted using an acidic pH or more gently by competitive elution using free Spot peptide. The high stability of the anti-Spot-Nanobody is reflected by an extraordinarily robust performance of the Spot-Trap in the presence of detergents, chaotropic agents, high salt concentrations or extreme pH. Even multiple regeneration cycles for repeated use of the Spot-Trap are possible.
- Harsh washing conditions
- Native & non-native elution
- High affinity
CRISPR/Cas
Undoubtedly, the Spot-Tag is a valuable tool for studying heterologously overexpressed proteins. However, by introducing the Spot-Tag using the CRISPR/Cas9 technology, proteins can be studied at the endogenous expression level. Using both Spot-Trap and Spot-Label, endogenous Spot-tagged protein from a CRISPR/Cas9-engineered cell line was used for immunoprecipitation and Western blotting as well as for immunofluorescence applications.
Immunofluorescence
Spot-tagged proteins can be detected by immunofluorescence using various microscopy techniques, including epi-fluorescence, confocal, and super resolution microscopy. ChromoTek’s Spot-Label offers a choice of different fluorophores for detection. Spot-Label is the first Nanobody used in super resolution microscopy for the detection of a peptide tag. Because of its small size, Spot-Label has a minimal epitope-to-label displacement, which leads to better resolution. In addition, the small size allows excellent tissue penetration.
- Higher resolution
- Better epitope access & tissue penetration
- Very small probe size
Immunoprecipitation
Spot-tagged proteins can be immunoprecipitated using Spot-Trap. The Spot-Tag can be placed either at the N-terminus or at the C-terminus of the protein of interest; also, it can be placed at internal positions of the protein, e.g. in flexible loops. Spot-Trap is available both as agarose beads or as magnetic agarose beads and is characterized by a remarkably low unspecific background binding.
- No contaminating heavy & light chains observed at pulldowns with conventional antibodies
- Harsh washing conditions
- High affinity
Co-IP Mass spectrometry
Interactome studies can be performed using Spot-Trap for co-immunoprecipitations followed by mass spectrometric analysis. Trypsinization (i.e. the proteolytic digest of the protein sample with trypsin) can be done directly on the beads, without elution of the immunoprecipitated proteins. Due to the small size of the anti-Spot-Nanobody, only four to five defined Nanobody-derived peptides are generated during the on-bead trypsinization.
Western blotting
The mouse monoclonal Spot-tag antibody [28A5], can be used in Western blotting for the detection of Spot-tagged fusion proteins. The anti-Spot-tag antibody [28A5] can be detected with the Nano-Secondary® alpaca anti-mouse IgG1, recombinant VHH, Alexa Fluor® 488 [CTK0103, CTK0104].
Expression of Spot-tagged proteins
For the expression of Spot-tagged fusion proteins use Spot-Tag vectors. A selection of vectors is available. Alternatively, Spot-Tag can be easily cloned by PCR primers.
Test sample
Please contact support@chromotek.com for a test sample.