Biomarkers for neurodegenerative disorders
Relevant fluid biomarkers are required to enable early and accurate diagnosis of neurodegenerative diseases. This blog details current biomarkers and promising future biomarkers.
Written by Nerea Gómez de San José, PhD student at the Institute of Experimental Neurology, Ulm University Hospital.
Neurodegenerative diseases, such as Alzheimer’s disease (AD), frontotemporal lobar degeneration (FTLD), and Lewy body dementia (LBD), are a group of disorders characterized by the progressive loss of structure and function of neurons, resulting in the impairment of the central nervous system. Moreover, these diseases are characterized by the accumulation of misfolded proteins, known as proteinopathy, and neuroinflammation (1). The degeneration of specific brain regions leads to different clinical features such as behavioral symptoms or parkinsonism. The primary risk factor for the development of neurodegenerative disorders is aging (2). Due to increasing life expectancy, the prevalence of many of these disorders is expected to rise significantly in the coming decades (3). Therefore, relevant biomarkers are needed to enable early and accurate diagnosis, improve treatment strategies, and support clinical trials in search of effective disease-modifying therapies (4). In recent years, significant progress has led to the identification of fluid biomarkers in cerebrospinal fluid (CSF) and blood.
Cerebrospinal fluid biomarkers for neurodegenerative diseases
CSF, the fluid surrounding the central nervous system, is an ideal source of biomarkers for neurodegenerative diseases. Its direct contact with the extracellular space of the brain and spinal cord offers insights into the biochemical changes occurring in these disorders. Among CSF biomarkers, core AD biomarkers are the most well-established and are now being introduced into clinical practice. Amyloid-β, phosphorylated tau-181 (p-tau181), and total tau serve as markers of amyloid pathology, tau pathology, and overall neurodegeneration, respectively, and have the potential to facilitate the monitoring of emerging AD treatments (5). However, there are still no biomarkers for AD diagnosis in the presymptomatic phase or to monitor other clinical symptoms, such as cognitive decline.
Apart from the previously mentioned AD biomarkers, there is currently a lack of diagnostic markers for other neurodegenerative disorders, such as FTLD or LBD (6,7). FTLD is a highly heterogeneous spectrum of diseases, leading to significant challenges in discovering biomarkers (8). In addition, α-synuclein, the protein that aggregates in the brain of LBD patients, is decreased in CSF. However, the high overlap with controls makes its implementation in the clinical routine difficult (9). Neurofilament light (NfL) is accepted as a biomarker of overall neurodegeneration and axonal damage. However, it lacks potential as a differential diagnostic marker as it is increased in ALS, FTD, atypical parkinsonian disorders, and AD (4,10). Biomarkers reflecting other pathological changes occurring in neurodegenerative disorders, such as synaptic loss (e.g., neurogranin, SNAP-25, GAP-43, and NPTX) or glial activation (GFAP), are currently under investigation (4,11,12).
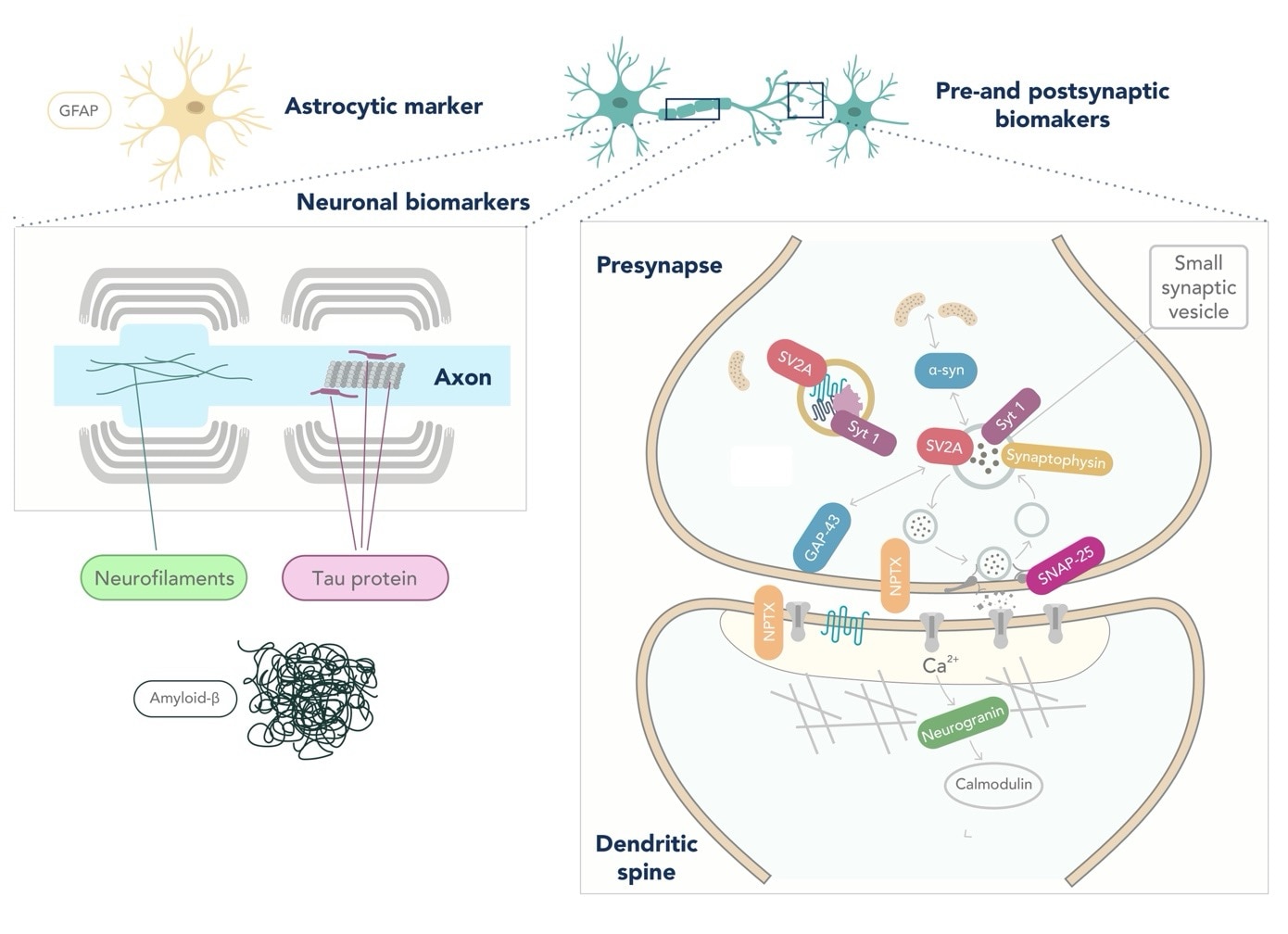
Figure: Location of popular fluid biomarkers for Neurodegenerative diseases. Figure adapted from Camporesi et al, 2020 (12).
Blood biomarkers for neurodegenerative diseases
Despite the advantages of CSF mentioned above, the collection procedure is painful and invasive, thus limiting its potential as a frequently sampled fluid for biomarker detection. There is an increased interest in developing blood-based biomarkers that will allow population screening. However, the analysis of brain-derived proteins in blood is a challenging process. Proteins must cross the blood-brain barrier and access the bloodstream through the lymphatic system. Once in the blood, the intricate composition of plasma poses a challenge for the accurate detection of brain-derived proteins. In addition, the expression of brain biomarkers in peripheral organs makes it challenging to distinguish between changes caused by CNS events from those in the periphery. The development of highly sensitive methods, such as Simoa®, EllaTM, or ElectroChemiLuminiscence (ECL), has enabled the detection of blood biomarkers such as amyloid-β, p-tau181, or NfL with the potential to become screening biomarkers of Aβ pathology and overall neurodegeneration (13). Moreover, biomarkers such as GFAP (14) and β-synuclein (15) are emerging as potential blood biomarkers and could unveil further pathological mechanisms or aid in the early diagnosis of these diseases.
Techniques for biomarker measurement
Two main approaches exist for the discovery of new candidates’ biomarkers: (1) prior knowledge of proteins involved in the pathological mechanism of the disease, and (2) discovery approaches using non-targeted methods that involve unbiased analysis of large sets of proteins. These explorative approaches include the widely used mass spectrometry and newly developed multiplexed antibody-based methods (e.g., proximity extension assay) (16). Once new candidate biomarkers have potentially been identified, targeted and quantitative methods are required to fully validate them. Targeted MS techniques and classical antibody binding assays such as enzyme-linked immunosorbent assay (ELISA) are used (16). ELISA permits an easier implementation into a clinical routine but requires highly sensitive and specific antibodies. Alternatively, real-time quaking-induced conversion (RT-QuIC) technology is currently used to detect misfolded proteins such as CSF α-synuclein, which could be a future diagnostic strategy for other proteins (17).
Challenges and future perspectives
Despite the tremendous progress in biomarker discovery over the last few decades and the establishment of several biomarkers for the diagnosis of AD, the development of biomarkers for other neurodegenerative disorders lags significantly behind. Several challenges still need to be addressed to enable further development in this field. One major obstacle is the limited understanding of the pathophysiological mechanisms underlying these disorders, which could hinder the identification of new candidate biomarkers. The high complexity and heterogeneity of many diseases further complicate the discovery of a single candidate biomarker that can reliably enable patient diagnosis. In such cases, a panel of biomarkers may be necessary. Furthermore, the availability of high-quality and well-characterized patient samples, high-quality reagents, reference materials, and proper analytical validation are essential to ensure reproducible results (18).
Recently developed, highly sensitive techniques and machine learning algorithms have unveiled promising opportunities for the discovery and clinical implementation of blood-based biomarkers, offering bright prospects for the future. Biomarkers could improve patient diagnosis and treatment, leading to a more personalized healthcare approach.
Antibodies and biomarkers:
ELISA kits
References
- Wilson DM, Cookson MR, Van Den Bosch L, Zetterberg H, Holtzman DM, Dewachter I. Hallmarks of neurodegenerative diseases. Cell. 2023 Feb;186(4):693–714.
- Hou Y, Dan X, Babbar M, Wei Y, Hasselbalch SG, Croteau DL, et al. Ageing as a risk factor for neurodegenerative disease. Nat Rev Neurol. 2019 Oct;15(10):565–81.
- International AD, Wimo A, Ali GC, Guerchet M, Prince M, Prina M, et al. World Alzheimer Report 2015: The global impact of dementia: An analysis of prevalence, incidence, cost and trends. 2015 Sep 21 [cited 2023 May 3]; Available from: https://www.alzint.org/resource/world-alzheimer-report-2015/
- Hansson O. Biomarkers for neurodegenerative diseases. Nat Med. 2021 Jun;27(6):954–63.
- Simrén J, Elmgren A, Blennow K, Zetterberg H. Chapter Six - Fluid biomarkers in Alzheimer’s disease. In: Makowski GS, editor. Advances in Clinical Chemistry [Internet]. Elsevier; 2023 [cited 2023 Jul 2]. p. 249–81. Available from: https://www.sciencedirect.com/science/article/pii/S0065242322000749
- McKeith IG, Boeve BF, Dickson DW, Halliday G, Taylor JP, Weintraub D, et al. Diagnosis and management of dementia with Lewy bodies: Fourth consensus report of the DLB Consortium. Neurology. 2017 Jul 4;89(1):88–100.
- del Campo M, Zetterberg H, Gandy S, Onyike CU, Oliveira F, Udeh-Momoh C, et al. New developments of biofluid-based biomarkers for routine diagnosis and disease trajectories in frontotemporal dementia. Alzheimers Dement. 2022;18(11):2292–307.
- Bang J, Spina S, Miller BL. Frontotemporal dementia. The Lancet. 2015 Oct 24;386(10004):1672–82.
- Parnetti L, Gaetani L, Eusebi P, Paciotti S, Hansson O, El-Agnaf O, et al. CSF and blood biomarkers for Parkinson’s disease. Lancet Neurol. 2019 Jun;18(6):573–86.
- Ashton NJ, Janelidze S, Al Khleifat A, Leuzy A, van der Ende EL, Karikari TK, et al. A multicentre validation study of the diagnostic value of plasma neurofilament light. Nat Commun. 2021 Jun 7;12(1):3400.
- Abdelhak A, Foschi M, Abu-Rumeileh S, Yue JK, D’Anna L, Huss A, et al. Blood GFAP as an emerging biomarker in brain and spinal cord disorders. Nat Rev Neurol. 2022 Mar;18(3):158–72.
- Camporesi E, Nilsson J, Brinkmalm A, Becker B, Ashton NJ, Blennow K, et al. Fluid Biomarkers for Synaptic Dysfunction and Loss. Biomark Insights. 2020 Jan 1;15:1177271920950319.
- Ashton NJ, Hye A, Rajkumar AP, Leuzy A, Snowden S, Suárez-Calvet M, et al. An update on blood-based biomarkers for non-Alzheimer neurodegenerative disorders. Nat Rev Neurol. 2020 May;16(5):265–84.
- Heller C, Foiani MS, Moore K, Convery R, Bocchetta M, Neason M, et al. Plasma glial fibrillary acidic protein is raised in progranulin-associated frontotemporal dementia. J Neurol Neurosurg Psychiatry. 2020 Mar;91(3):263–70.
- Oeckl P, Janelidze S, Halbgebauer S, Stomrud E, Palmqvist S, Otto M, et al. Higher plasma β-synuclein indicates early synaptic degeneration in Alzheimer’s disease. Alzheimers Dement J Alzheimers Assoc. 2023 Apr 27;
- Waury K, Willemse EAJ, Vanmechelen E, Zetterberg H, Teunissen CE, Abeln S. Bioinformatics tools and data resources for assay development of fluid protein biomarkers. Biomark Res. 2022 Nov 15;10(1):83.
- Groveman BR, Orrù CD, Hughson AG, Raymond LD, Zanusso G, Ghetti B, et al. Rapid and ultra-sensitive quantitation of disease-associated α-synuclein seeds in brain and cerebrospinal fluid by αSyn RT-QuIC. Acta Neuropathol Commun. 2018 Feb 9;6(1):7.
- van Gool AJ, Bietrix F, Caldenhoven E, Zatloukal K, Scherer A, Litton JE, et al. Bridging the translational innovation gap through good biomarker practice. Nat Rev Drug Discov. 2017 Sep;16(9):587–8.
Related Content
7 Tips to Successfully Culture Primary Rodent Neurons
Support
Newsletter Signup
Stay up-to-date with our latest news and events. New to Proteintech? Get 10% off your first order when you sign up.
