PCSK9 Rekombinanter Antikörper
PCSK9 Rekombinant Antikörper für WB, IF/ICC, FC (Intra), Sandwich ELISA, Indirect ELISA, Sample test
Wirt / Isotyp
Kaninchen / IgG
Getestete Reaktivität
human
Anwendung
WB, IF/ICC, FC (Intra), Sandwich ELISA, Indirect ELISA, Sample test
Konjugation
Unkonjugiert
CloneNo.
241395G1
Kat-Nr. : 84172-4-PBS
Synonyme
Geprüfte Anwendungen
Produktinformation
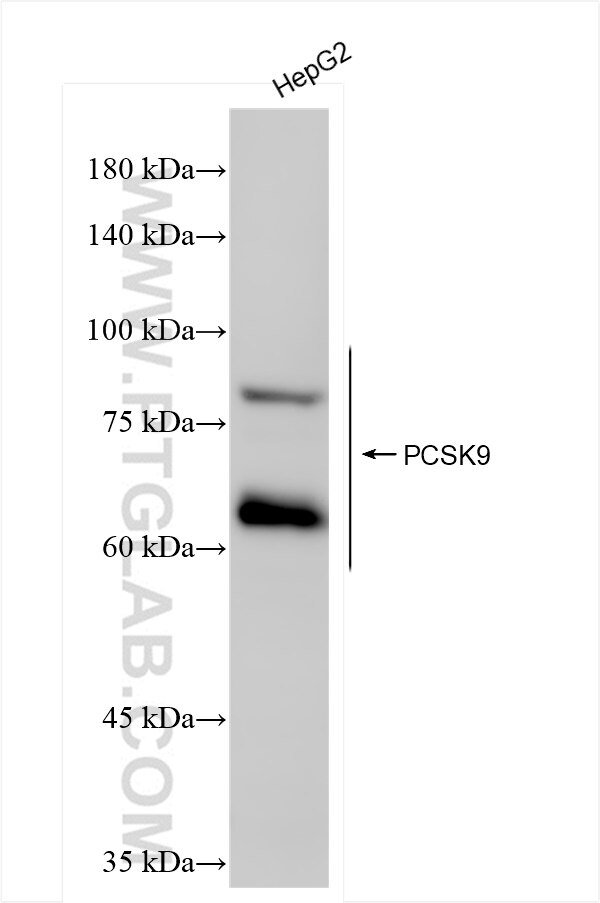
84172-4-PBS bindet in WB, IF/ICC, FC (Intra), Sandwich ELISA, Indirect ELISA, Sample test PCSK9 und zeigt Reaktivität mit human
| Getestete Reaktivität | human |
| Wirt / Isotyp | Kaninchen / IgG |
| Klonalität | Rekombinant |
| Typ | Antikörper |
| Immunogen | PCSK9 fusion protein Eg0362 |
| Vollständiger Name | proprotein convertase subtilisin/kexin type 9 |
| Berechnetes Molekulargewicht | 74 kDa |
| Beobachtetes Molekulargewicht | 72-78 kDa, 62 kDa |
| GenBank-Zugangsnummer | NM_174936.4 |
| Gene symbol | PCSK9 |
| Gene ID (NCBI) | 255738 |
| Konjugation | Unkonjugiert |
| Form | Liquid |
| Reinigungsmethode | Protein-A-Reinigung |
| Lagerungspuffer | PBS only |
| Lagerungsbedingungen | Store at -80°C. 20ul Größen enthalten 0,1% BSA. |
Hintergrundinformationen
Proprotein convertase subtilisin/kexin type 9 (PCSK9) is a crucial protein governing the circulating levels of low density lipoprotein-cholesterol (LDL-C), by virtue of its pivotal role in the degradation of the LDL receptor (LDLR). PCSK9 is expressed in the kidney and lung. It is synthesized as a 72 kDa immature precursor that undergoes autocatalytic cleavage in the endoplasmic reticulum to generate a 63 kDa mature protein. The cleaved N-terminal fragment remains associated with the mature protein and is necessary for its secretion, allowing it to circulate in the blood. The ability of PCSK9 to regulate a diverse group of cell-surface proteins hinted that it might also be able to influence additional membrane proteins that are important in anti-tumour immune responses. Targeting PCSK9 to treat cancer is also attractive because two neutralizing antibodies against it, evolocumab and alirocumab, have already been approved for human clinical use to lower cholesterol levels. (PMID: 30522786, PMID: 22493497)