Tested Applications
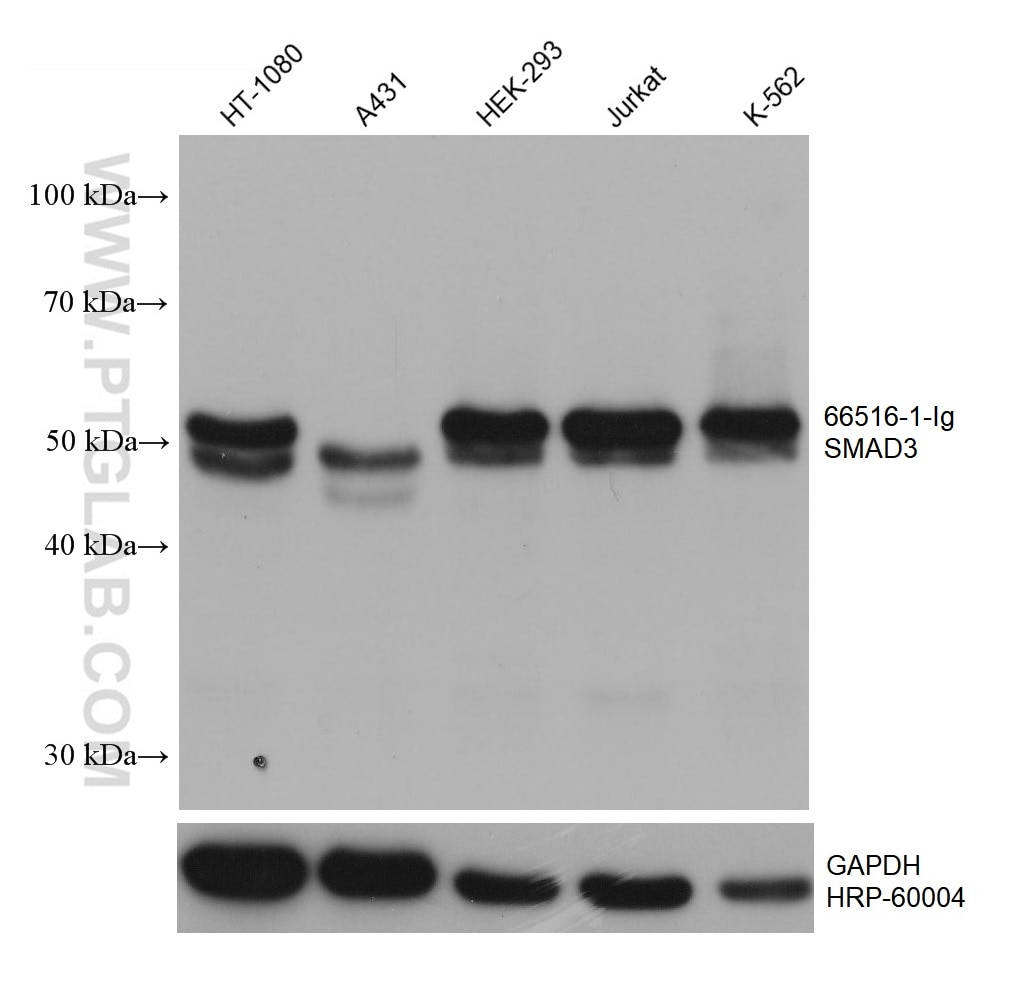
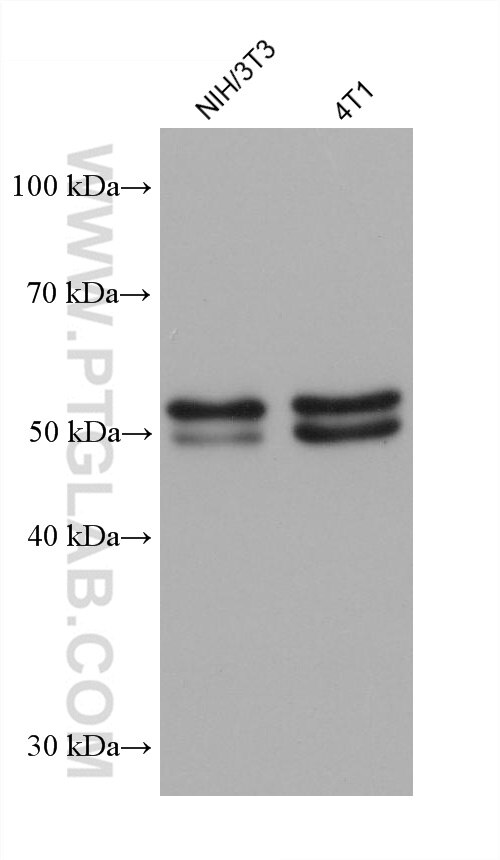
| Positive WB detected in | HT-1080 cells, NIH/3T3 cells, A431 cells, HEK-293 cells, Jurkat cells, K-562 cells, 4T1 cells |
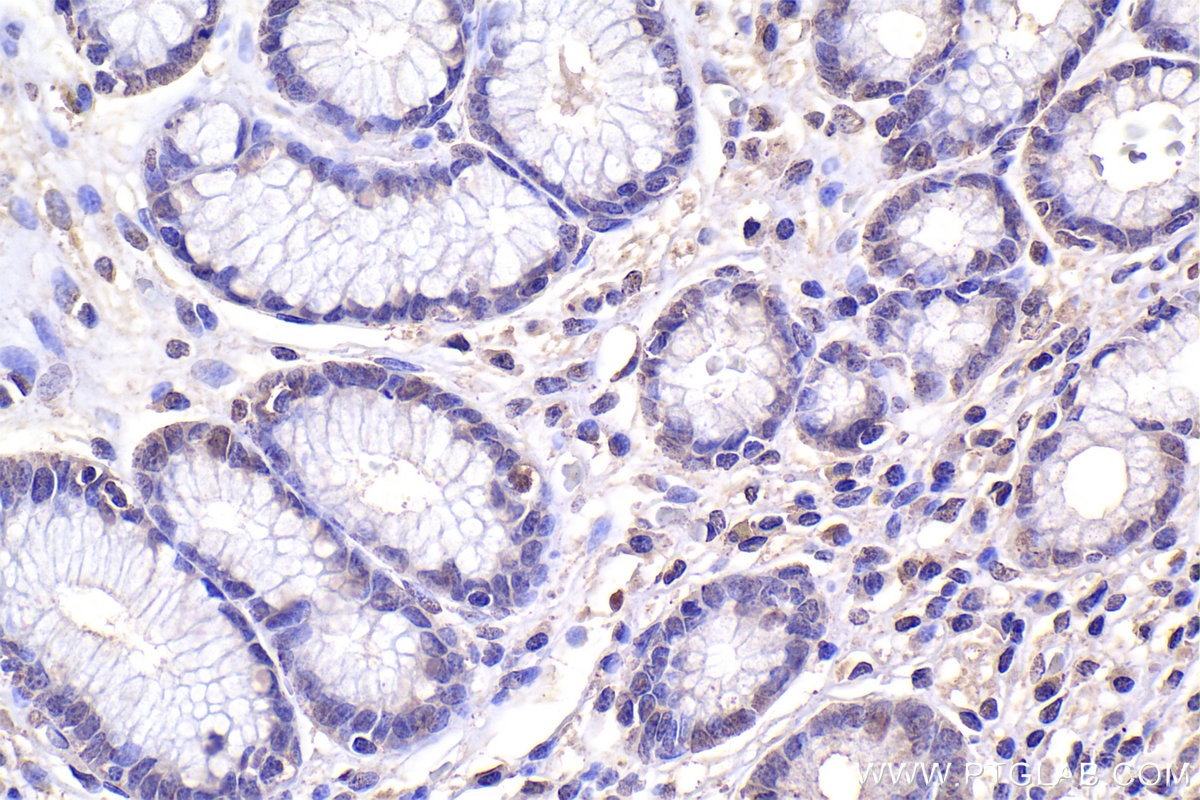
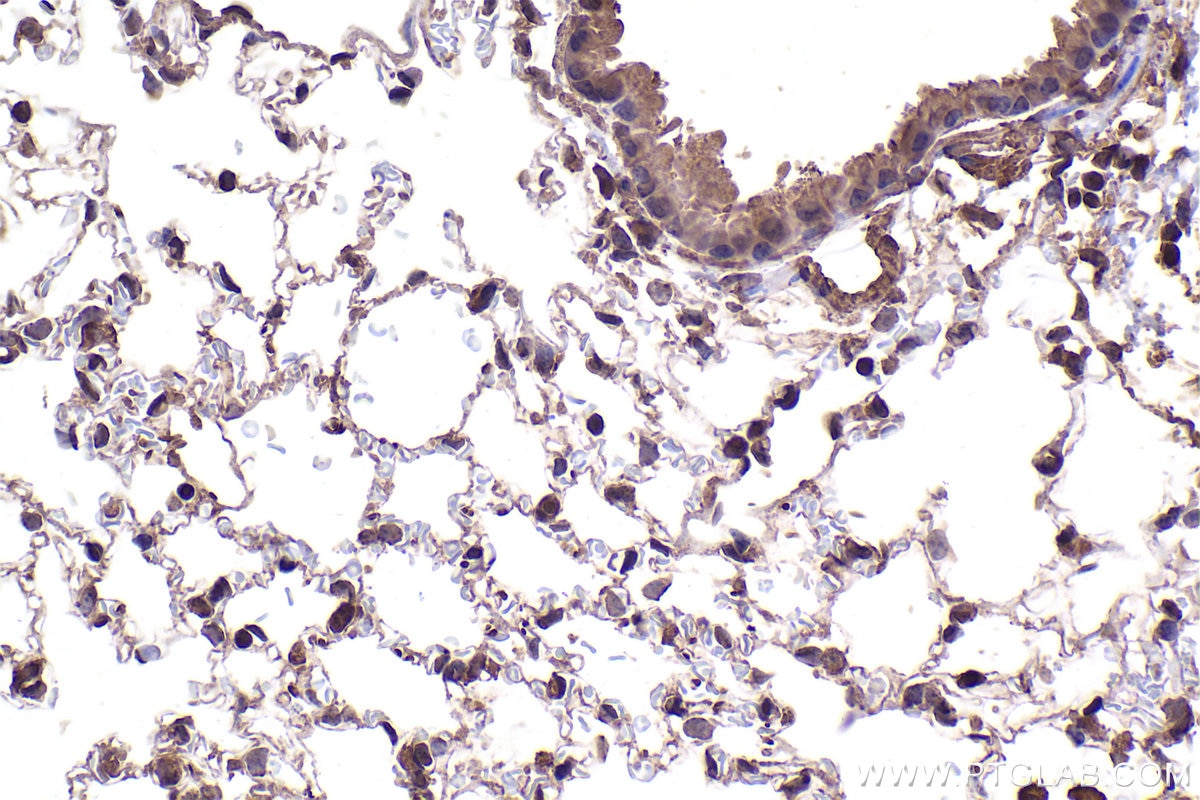
| Positive IHC detected in | human stomach cancer tissue, mouse lung tissue Note: suggested antigen retrieval with TE buffer pH 9.0; (*) Alternatively, antigen retrieval may be performed with citrate buffer pH 6.0 |
Recommended dilution
| Application | Dilution |
|---|---|
| Western Blot (WB) | WB : 1:2000-1:12000 |
| Immunohistochemistry (IHC) | IHC : 1:500-1:2000 |
| It is recommended that this reagent should be titrated in each testing system to obtain optimal results. | |
| Sample-dependent, Check data in validation data gallery. | |
Published Applications
| WB | See 117 publications below |
| IHC | See 3 publications below |
| IF | See 8 publications below |
| IP | See 1 publications below |
| CoIP | See 4 publications below |
| ChIP | See 1 publications below |
Product Information
66516-1-Ig targets SMAD3 in WB, IHC, IF, IP, CoIP, ChIP, ELISA applications and shows reactivity with human, mouse samples.
| Tested Reactivity | human, mouse |
| Cited Reactivity | human, mouse, rabbit |
| Host / Isotype | Mouse / IgG1 |
| Class | Monoclonal |
| Type | Antibody |
| Immunogen |
Peptide Predict reactive species |
| Full Name | SMAD family member 3 |
| Calculated Molecular Weight | 48 kDa |
| Observed Molecular Weight | 55-60 kDa |
| GenBank Accession Number | NM_005902 |
| Gene Symbol | SMAD3 |
| Gene ID (NCBI) | 4088 |
| RRID | AB_2881879 |
| Conjugate | Unconjugated |
| Form | Liquid |
| Purification Method | Protein G purification |
| UNIPROT ID | P84022 |
| Storage Buffer | PBS with 0.02% sodium azide and 50% glycerol, pH 7.3. |
| Storage Conditions | Store at -20°C. Stable for one year after shipment. Aliquoting is unnecessary for -20oC storage. 20ul sizes contain 0.1% BSA. |
Background Information
SMAD3, also named as hMAD 3 or Mad3, is a 425 amino acid protein, which contains one MH1 domain and one MH2 domain. SMAD3 localizes in the nucleus and cytoplasm. SMAD3 plays an essential role in development and maintenance of self-tolerance and is a critical mediator of the TGFB signaling pathway. SMAD3 is involved in TGFB dependent regulation of steroidogenesis and in T-cell response to TGFB.
Protocols
| Product Specific Protocols | |
|---|---|
| IHC protocol for SMAD3 antibody 66516-1-Ig | Download protocol |
| WB protocol for SMAD3 antibody 66516-1-Ig | Download protocol |
| Standard Protocols | |
|---|---|
| Click here to view our Standard Protocols |
Publications
| Species | Application | Title |
|---|---|---|
J Exp Clin Cancer Res ACTN1 promotes HNSCC tumorigenesis and cisplatin resistance by enhancing MYH9-dependent degradation of GSK-3β and integrin β1-mediated phosphorylation of FAK | ||
Drug Des Devel Ther Keloid Patient Plasma-Derived Exosomal hsa_circ_0020792 Promotes Normal Skin Fibroblasts Proliferation, Migration, and Fibrogenesis via Modulating miR-193a-5p and Activating TGF-β1/Smad2/3 Signaling | ||
Cancer Lett Direct contact between tumor cells and platelets initiates a FAK-dependent F3/TGF-β positive feedback loop that promotes tumor progression and EMT in osteosarcoma | ||
Cell Chem Biol Substrate-dependent interaction of SPOP and RACK1 aggravates cardiac fibrosis following myocardial infarction | ||
BMC Med Molecular landscape of atherosclerotic plaque progression: insights from proteomics, single-cell transcriptomics and genomics | ||
Cell Mol Life Sci Macrophage Dectin-1 mediates Ang II renal injury through neutrophil migration and TGF-β1 secretion |
Reviews
The reviews below have been submitted by verified Proteintech customers who received an incentive for providing their feedback.
FH Juliette (Verified Customer) (11-25-2024) | Good
|