Recombinant Mouse uPAR/CD87 protein (rFc Tag)
Species
Mouse
Purity
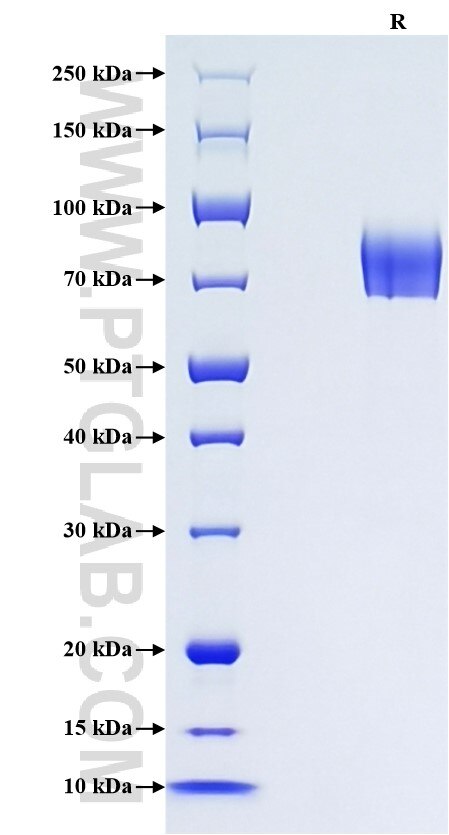
>90 %, SDS-PAGE
Tag
rFc Tag
Activity
not tested
Cat no : Eg3156
Validation Data Gallery
Product Information
| Purity | >90 %, SDS-PAGE |
| Endotoxin | <0.1 EU/μg protein, LAL method |
| Activity |
Not tested |
| Expression | HEK293-derived Mouse uPAR protein Leu24-Thr297 (Accession# P35456-1) with a rabbit IgG Fc tag at the C-terminus. |
| GeneID | 18793 |
| Accession | P35456-1 |
| PredictedSize | 56.0 kDa |
| SDS-PAGE | 65-90 kDa, reducing (R) conditions |
| Formulation | Lyophilized from 0.22 μm filtered solution in PBS, pH 7.4. Normally 5% trehalose and 5% mannitol are added as protectants before lyophilization. |
| Reconstitution | Briefly centrifuge the tube before opening. Reconstitute at 0.1-0.5 mg/mL in sterile water. |
| Storage Conditions |
It is recommended that the protein be aliquoted for optimal storage. Avoid repeated freeze-thaw cycles.
|
| Shipping | The product is shipped at ambient temperature. Upon receipt, store it immediately at the recommended temperature. |
Background
uPAR/CD87 is a highly glycosylated GPI-anchored membrane protein. In addition to the membrane-anchored form, uPAR is released from the plasma membrane by cleavage of the GPI anchor and can be found as a soluble form (suPAR). uPAR contains three homologous domains (D1-D3) of which the N-terminal one (D1) represents the uPA-binding domain. After binding to uPAR, uPA cleaves plasminogen, generating the active protease plasmin which is involved in a wide variety of physiologic and pathologic processes. In addition to regulating proteolysis, uPAR has important function in cell adhesion, migration and proliferation. Studies reveal that uPAR expression is elevated during inflammation and tissue remodelling and in many human cancers, in which it frequently indicates poor prognosis. suPAR has been detected in plasma, and increased plasma concentrations of suPAR have been found in patients with some advanced cancers.
References:
1. Ploug M. et al. (1991). J Biol Chem. 266(3):1926-1933. 2. Stephens RW. et al. (1997). Clin Chem. 43(10):1868-1876. 3. Blasi F. et al. (2002). Nat Rev Mol Cell Biol. 3(12):932-943. 4. Smith HW. et al. (2010). Nat Rev Mol Cell Biol. 11(1):23-36.