Recombinant Human KIR2DL3 protein (rFc Tag) (HPLC verified)
Species
Human
Purity
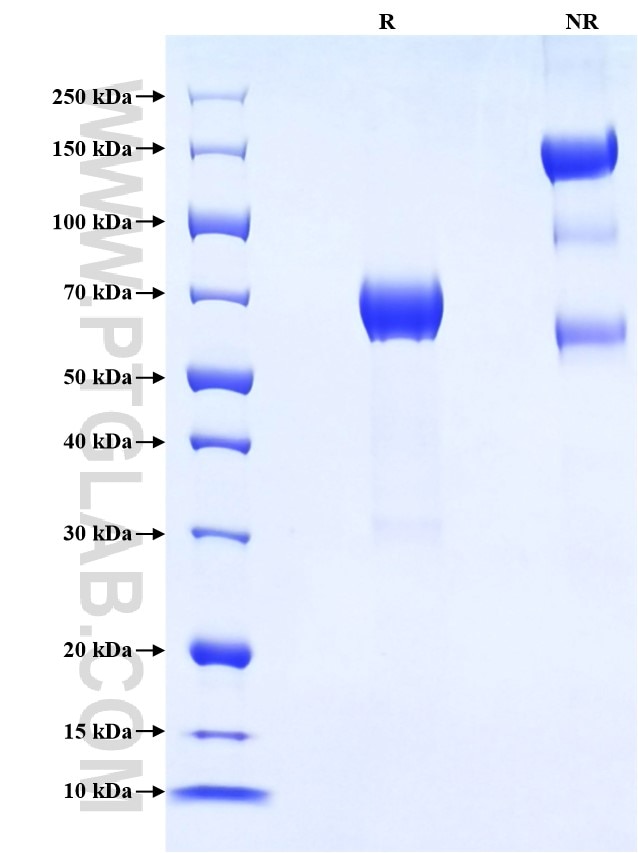
>90 %, SDS-PAGE
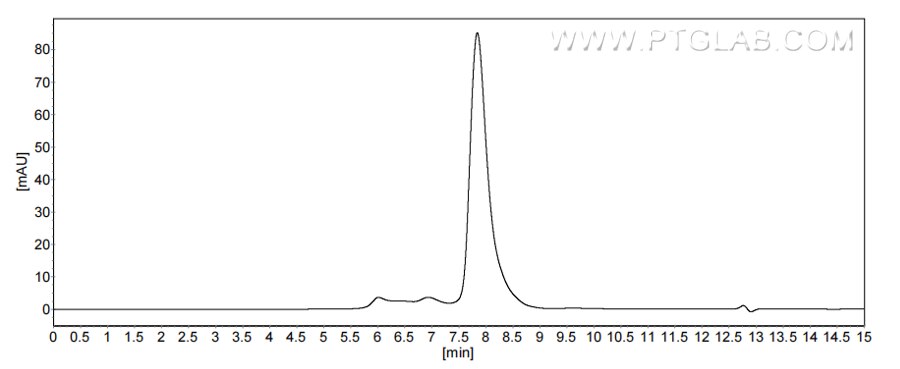
>90 %, SEC-HPLC
Tag
rFc Tag
Activity
not tested
Cat no : Eg2699
Validation Data Gallery
Product Information
| Purity | >90 %, SDS-PAGE >90 %, SEC-HPLC |
| Endotoxin | <0.1 EU/μg protein, LAL method |
| Activity |
Not tested |
| Expression | HEK293-derived Human KIR2DL3 protein His22-His245 (Accession# P43628-1) with a rabbit IgG Fc tag at the C-terminus. |
| GeneID | 3804 |
| Accession | P43628-1 |
| PredictedSize | 50.6 kDa |
| SDS-PAGE | 60-75 kDa, reducing (R) conditions |
| Formulation | Lyophilized from 0.22 μm filtered solution in PBS, pH 7.4. Normally 5% trehalose and 5% mannitol are added as protectants before lyophilization. |
| Reconstitution | Briefly centrifuge the tube before opening. Reconstitute at 0.1-0.5 mg/mL in sterile water. |
| Storage Conditions |
It is recommended that the protein be aliquoted for optimal storage. Avoid repeated freeze-thaw cycles.
|
| Shipping | The product is shipped at ambient temperature. Upon receipt, store it immediately at the recommended temperature. |
Background
Killer cell immunoglobulin-like receptors (KIRs) are a diverse family of inhibitory and activating receptors expressed on NK cells and a subset of T cells. These polymorphic receptors interact with specific motifs on HLA class I molecules, modulate NK cytolytic activity, and are encoded by genes located on chromosome 19q13.4. KIR2DL3, also known as CD158B2 or NKAT2, is an inhibitory receptor that is specific for HLA-C alleles (HLA-Cw1, HLA-Cw3, and HLA-Cw7). It is a 341-amino acid transmembrane glycoprotein consisting of an extracellular region containing two C2-type Ig-like domains, a 19-amino acid hydrophobic transmembrane region, and a long cytoplasmic tail with two immunoreceptor tyrosine-based inhibitory motifs (ITIMs). KIR2DL3 inhibits the cytolytic activity of NK cells thus preventing cell lysis.
References:
1. Carlos Vilches. et al. (2002). Annu Rev Immunol. 20:217-251. 2. A Bontadini. et al. (2006). J Transl Med. 4:44. 3. K Maenaka. et al. (1999). Structure. 7(4):391-398. 4. M Vitale. et al. (1995). Proc Natl Acad Sci U S A. 92(8):3536-3540.