Abstract on Nano-BiTEs: Bispecific T cell engagers based on nanobodies
A novel format for bispecific T cell engagers
Bispecific T cell engagers (BiTEs) mediate the killing of tumour cells by recruiting and activating T cells. BiTEs usually consist out of two scFv units fused by a short linker. One scFv targets the T cell receptor (TCR) subunit CD3ε, the other scFv a tumour-associated antigen.
By binding both to a TCR and a tumour antigen, a BiTE molecule is thus able (1) to guide a T cell to a tumour cell and (2) to activate this T cell by mimicking an immunological synapse, independently of the actual TCR specificity.
Here, we propose a novel format for bispecific T cell engagers, which we dub Nano-BiTEs. They comprise a nanobody against a tumour antigen fused to a well-established anti-CD3ε scFv. Nanobodies (also called VHHs) are highly stable single-domain antibodies derived from camelid heavy-chain-only antibodies (HCAbs) and figure among the smallest known antibody fragments (see graphic below).
We hypothesised that the incorporation of nanobodies would facilitate the production and stability of our Nano-BiTE format.
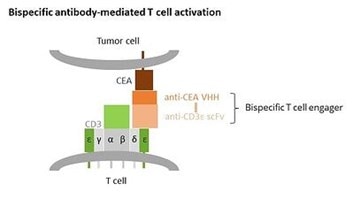
Bispecific T cell engager anti-CEA x anti-CD3ε
binds to CD3 on T cells and CEA on tumor cells
Currently, we have developed Nano-BiTEs specific for the tumour-associated antigens Her2 (also known as erbB-2 or Neu) and CEA (carcinoembryonic antigen). Both constructs were well expressed in mammalian cell culture and readily purified to homogeneity. Using flow cytometry and immunofluorescence microscopy, we confirmed binding of our Nano-BiTEs to Her2, CEA and CD3ε on cells. In vitro, these Nano-BiTEs bind to their respective targets with nanomolar affinities. Importantly, our Nano-BiTEs are able to bind concomitantly to both CD3ε and their respective tumour antigen, a hallmark of bispecific T cell engagers.
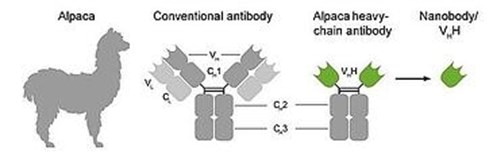
Origin of Nanobodies and comparison with conventional antibody
We anticipate that our Nano-BiTEs will activate T cells in the context of tumour cells in vitro and ex vivo. For example, they can be used as standards in the development of novel bispecific formats. Moreover, they can be used to achieve a defined level of T cell activation to quantify the effect of novel drug candidates (such as check-point modulators) given in addition. Thus, we expect that our Nano-Bites, as a novel tool format, will advance the burgeoning field of immuno-oncology.
