Immunoprecipitation without additional bands
Pull-down of proteins with Nano-Traps
Introduction
Immunoprecipitation (IP) is the pull-down of a protein of interest (POI) by an antibody that is immobilized on beads. While in the past antibodies against POIs have been used, nowadays IPs are also conducted using antibodies against fusion tags. One of the most popular fusion tags for IP is Green fluorescent protein (GFP).
Typical immunoprecipitation using conventional antibodies
Traditionally, for an immunoprecipitation the antibody is immobilized by protein A/G beads to pull down the POI from the cell lysate. The assay comprises 3 steps:
- Incubation of the antibody with cell lysate containing the target protein and the formation of the antibody:protein complex.
- Precipitation of the complex by binding to protein A/G beads.
- Elution from the beads with SDS sample buffer and analysis by SDS-PAGE and Western blotting.
Using this approach, antibodies against the protein of interest can be applied.
However, conventional antibodies have several drawbacks for immunoprecipitation, which are discussed below:
Heavy and light antibody chains appear on SDS-PAGE
 |
The interaction of the antibody to protein A/G is easily disrupted during the elution with SDS sample buffer. Therefore, the protein fraction contains not only the POI but also antibody heavy and light chains. These heavy and light chains (50 kDa and 25 kDa) also appear on the SDS-PAGE in addition to the POI band. |
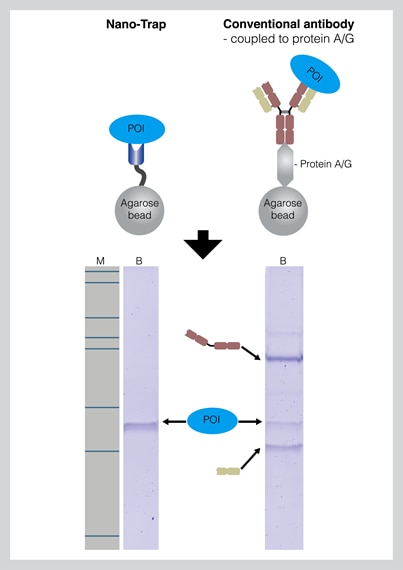
Antibody chains can be detected on a Western Blot
Heavy and/or light chains of the antibody used for IP, i.e. the IP antibody, may also be detected next to the POI by Western blotting. Two cases are distinguished:
- The IP antibody is the same species/subclass as the primary WB antibody. The secondary WB antibody may detect both the primary WB antibody bound to the target and the heavy and/or light chains of the IP antibody.
- The secondary WB antibody cross-reacts and may detect the IP antibody although the species/subclass is different to the primary WB antibody.
Note that sometimes only the heavy or the light chain are immunostained, which makes the interpretation of the results even harder.
The interpretation of Co-immunoprecipitation (Co-IP) data can become even more complicated, because prey and bait protein and antibody chain bands have to be correctly annotated.
Antibodies chains make the interpretation of MS data from Co-IPs difficult
The heavy and light antibody chains present in fractions from Co-IP assays can interfere with MS analysis, particularly when on-bead digestion is used. Here, the multiple peptides resulting from the antibody’s digest can lead to complex MS results that are difficult to interpret. Note, that on-bead digestion is the preferred method for a gentle and complete transfer of immunoprecipitated protein complexes to MS.
How to overcome these obstacles:
Use ChromoTek’s GFP-Trap – GFP Immunoprecipitation without antibody heavy and light chain contaminations
As outlined above, the presence of heavy and light chains of the IP antibody can affect the interpretation of IP results in several ways. Therefore, we recommend the use of Nanobody-based reagents instead. ChromoTek’s GFP-Trap consists of a 15 kDa single domain antibody (or Nanobody), that is conjugated to beads. This Nanobody remains on the beads and is virtually not eluted during elution with SDS sample buffer. Accordingly, the Nanobody doesn’t appear on SDS-PAGE or Western blot. Furthermore, GFP-Trap allows a free selection of primary and secondary Western Blot antibodies. For MS analysis after gentle on-bead digestion the small size Nanobody, which is only 1/10th of an antibody, results in just 4-7 additional peptides.
