Recombinant Human Serpin E1/PAI-1 protein (rFc Tag)
Species
Human
Purity
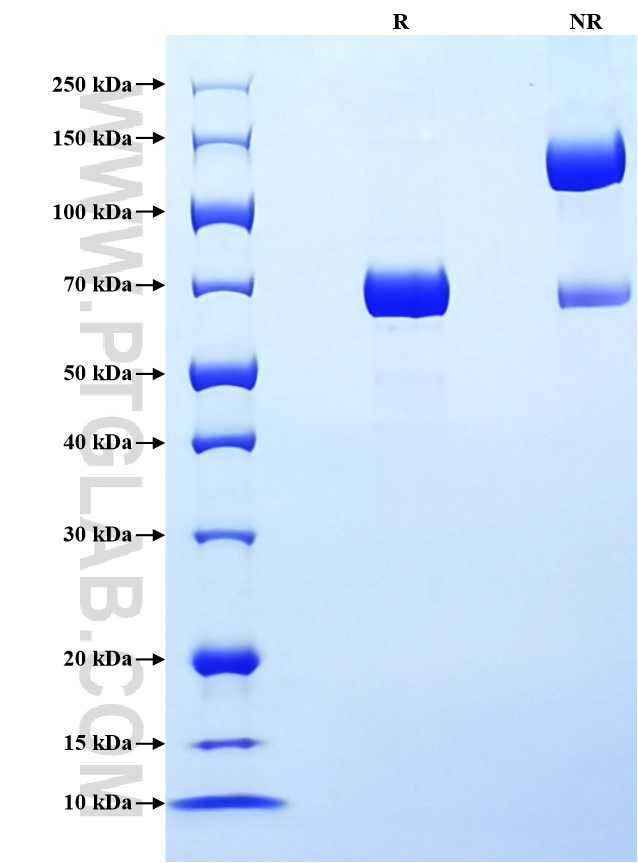
>90 %, SDS-PAGE
Tag
rFc Tag
Activity
not tested
Cat no : Eg3798
Validation Data Gallery
Product Information
| Purity | >90 %, SDS-PAGE |
| Endotoxin | <0.1 EU/μg protein, LAL method |
| Activity |
Not tested |
| Expression | HEK293-derived Human Serpin E1 protein Val24-Pro402 (Accession# P05121-1) with a rabbit IgG Fc tag at the N-terminus. |
| GeneID | 5054 |
| Accession | P05121-1 |
| PredictedSize | 70.0 kDa |
| SDS-PAGE | 65-75 kDa, reducing (R) conditions |
| Formulation | Lyophilized from 0.22 μm filtered solution in PBS, pH 7.4. Normally 5% trehalose and 5% mannitol are added as protectants before lyophilization. |
| Reconstitution | Briefly centrifuge the tube before opening. Reconstitute at 0.1-0.5 mg/mL in sterile water. |
| Storage Conditions |
It is recommended that the protein be aliquoted for optimal storage. Avoid repeated freeze-thaw cycles.
|
| Shipping | The product is shipped at ambient temperature. Upon receipt, store it immediately at the recommended temperature. |
Background
SERPINE1, also named as Plasminogen activator inhibitor type 1 (PAI-1), is a member of the serine protease inhibitor (SERPIN) superfamily. It is produced by the vascular endothelium, the liver, the monocytes/macrophagues, the platelets and the adipose tissue. High plasma levels of PAI-1 have been associated with an increased risk of suffering cardiovascular diseases. It is implicated in the pathogenesis of obesity, insulin resistance and type 2 diabetes. In several tumor types, SERPINE1 expression is up-regulated and it has been described as a poor prognostic marker. Besides its prognostic value, SERPINE1 expression has been validated as a marker for therapy decision making in patients with node-negative breast cancer. Defects in this gene are the cause of plasminogen activator inhibitor-1 deficiency (PAI-1 deficiency), and high concentrations of the gene product are associated with thrombophilia.
References:
1. Huang J. et al.(2012). Blood. 20: 4873-81. 2. Kohler HP. et al. (2000). N Engl J Med. 342: 1792-801. 3. Festa A. et al. (2006). Circulation. 113: 1753-9. 4. Look MP. et al. (2002). J Natl Cancer Inst. 16;94(2):116-28. 5. Ulisse S. et al. (2009). Curr Cancer Drug Targets. 9(1):32-71.