Recombinant Human FABP4 protein (His Tag)
Species
Human
Purity
>90 %, SDS-PAGE
Tag
His Tag
Activity
not tested
Cat no : Eg0880
Validation Data Gallery
Product Information
| Purity | >90 %, SDS-PAGE |
| Endotoxin | <0.1 EU/μg protein, LAL method |
| Activity |
Not tested |
| Expression | HEK293-derived Human FABP4 protein Cys2-Ala132 (Accession# P15090) with a His tag at the C-terminus. |
| GeneID | 2167 |
| Accession | P15090 |
| PredictedSize | 15.7 kDa |
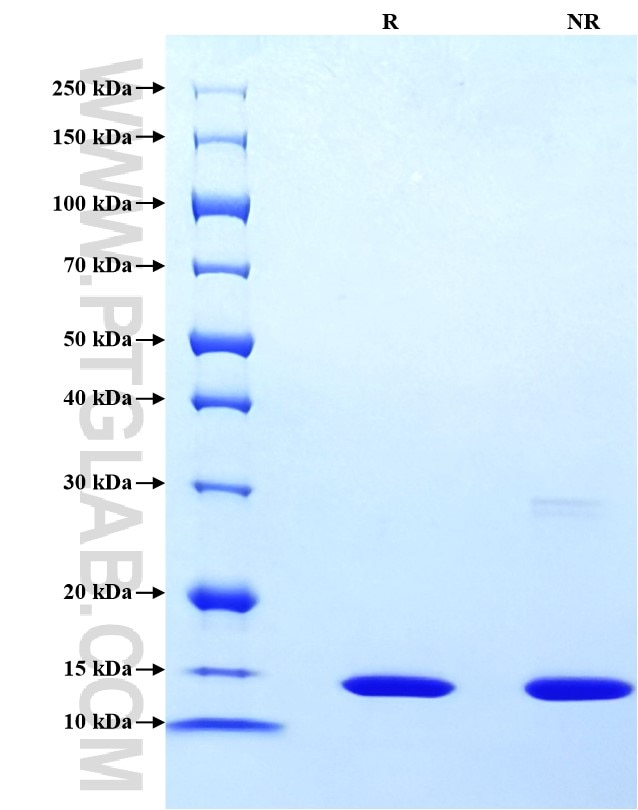
| SDS-PAGE | 13-15 kDa, reducing (R) conditions |
| Formulation | Lyophilized from 0.22 μm filtered solution in PBS, pH 7.4. Normally 5% trehalose and 5% mannitol are added as protectants before lyophilization. |
| Reconstitution | Briefly centrifuge the tube before opening. Reconstitute at 0.1-0.5 mg/mL in sterile water. |
| Storage Conditions |
It is recommended that the protein be aliquoted for optimal storage. Avoid repeated freeze-thaw cycles.
|
| Shipping | The product is shipped at ambient temperature. Upon receipt, store it immediately at the recommended temperature. |
Background
Fatty acid binding protein (FABP) 4 is a member of the FABP family which abundantly expressed, fatty acid carrier proteins. FABPs are capable of binding a variety of hydrophobic molecules such as long-chain fatty acids and are important for their uptake and intracellular trafficking. It was first identified as an adipocyte-specific protein, important for the maintenance of lipid and glucose metabolism. It is also detected in macrophages, where it participates in regulating inflammation and cholesterol trafficking via NFκB and PPAR. In more recent studies, FABP4 has been found in a variety of endothelial cells, where it has been identified as a target of VEGF and a regulator of cell proliferation and possibly angiogenesis. Pathologically, FABP4 has been associated with the development of metabolic syndrome, diabetes and cancer and vulnerability of atherosclerotic plaques. FABP4 has been identified as a novel prognostic factor for both adverse cardiovascular events and breast cancer.
References:
1.Lai W, et al. (2022)Eur J Pharmacol. 931:175224. 2.Floresta G, et al. (2022)Eur J Med Chem. 240:114604. 3.Furuhashi M, et al. (2014). Clin Med Insights Cardiol.8 (Suppl 3):23-33. 4.Furuhashi M. (2019) J Atheroscler Thromb. 26 (3):216-232.