Recombinant Human Nectin-4/PVRL4 protein (His Tag)
Species
Human
Purity
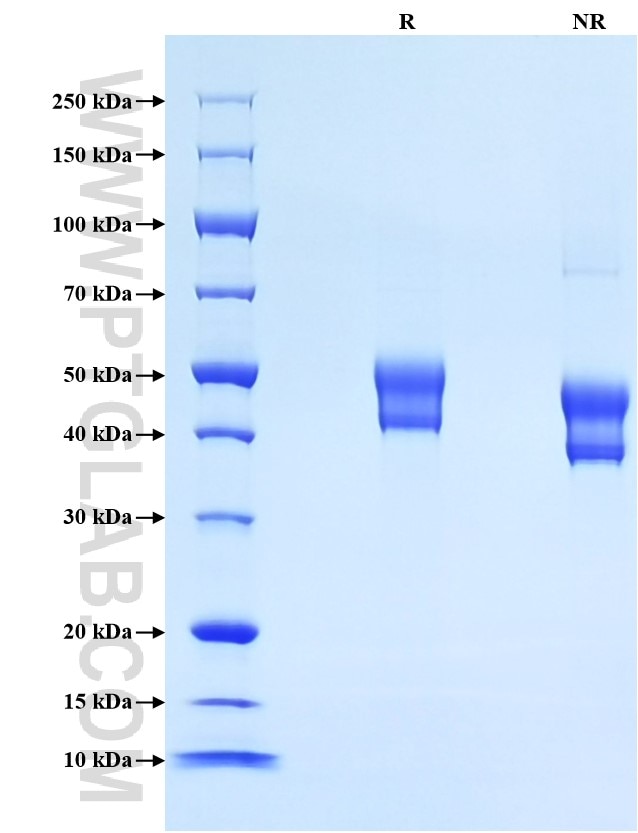
>90 %, SDS-PAGE
Tag
His Tag
Activity
not tested
Cat no : Eg0415
Validation Data Gallery
Product Information
| Purity | >90 %, SDS-PAGE |
| Endotoxin | <0.1 EU/μg protein, LAL method |
| Activity |
Not tested |
| Expression | HEK293-derived Human Nectin-4 protein Gly32-Ser349 (Accession# Q96NY8-1) with a His tag at the C-terminus. |
| GeneID | 81607 |
| Accession | Q96NY8-1 |
| PredictedSize | 37.9 kDa |
| SDS-PAGE | 40-52 kDa, reducing (R) conditions |
| Formulation | Lyophilized from 0.22 μm filtered solution in PBS, pH 7.4. Normally 5% trehalose and 5% mannitol are added as protectants before lyophilization. |
| Reconstitution | Briefly centrifuge the tube before opening. Reconstitute at 0.1-0.5 mg/mL in sterile water. |
| Storage Conditions |
It is recommended that the protein be aliquoted for optimal storage. Avoid repeated freeze-thaw cycles.
|
| Shipping | The product is shipped at ambient temperature. Upon receipt, store it immediately at the recommended temperature. |
Background
Nectin-4, also known as PVRL4, is a type-I transmembrane glycoprotein that belongs to the nectin subfamily of immunoglobulin-like adhesion molecules that participate in Ca(2+)-independent cell-cell adhesion. The extracellular domain of Nectin-4, which contains three immunoglobulin-like domains (V and two C2-type domains, VCC), can be proteolytically cleaved to release a soluble form. Nectin-4 interacts with afadin via its carboxyl-terminal cytoplasmic sequence and trans-interacts with nectin-1/PRR1 through V domain interaction. It acts as a receptor for measles virus. Nectin-4 is overexpressed in multiple human malignancies and the aberrant expression is correlated with cancer progression and poor prognostic.
References:
1. N Reymond, et al. (2001) J Biol Chem. 276(46):43205-15. 2. Stéphanie Fabre-Lafay. (2005) J Biol Chem. 280(20):19543-50. 3. Martin J Barron, et al. (2008) Hum Mol Genet. 17(22):3509-20. 4. Michael D Mühlebach, et al. (2011) Nature. 480(7378):530-3. 5. Wafa Bouleftour, et al. (2022) Mol Cancer Ther. 21(4):493-501.