Recombinant Human FCGR3A/CD16a (F176V) protein (Myc Tag, His Tag)
Species
Human
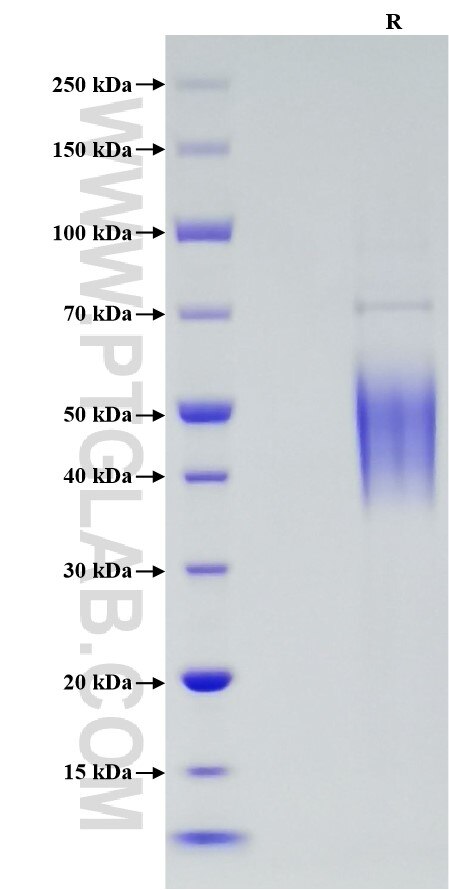
Purity
>90 %, SDS-PAGE
Tag
Myc Tag, His Tag
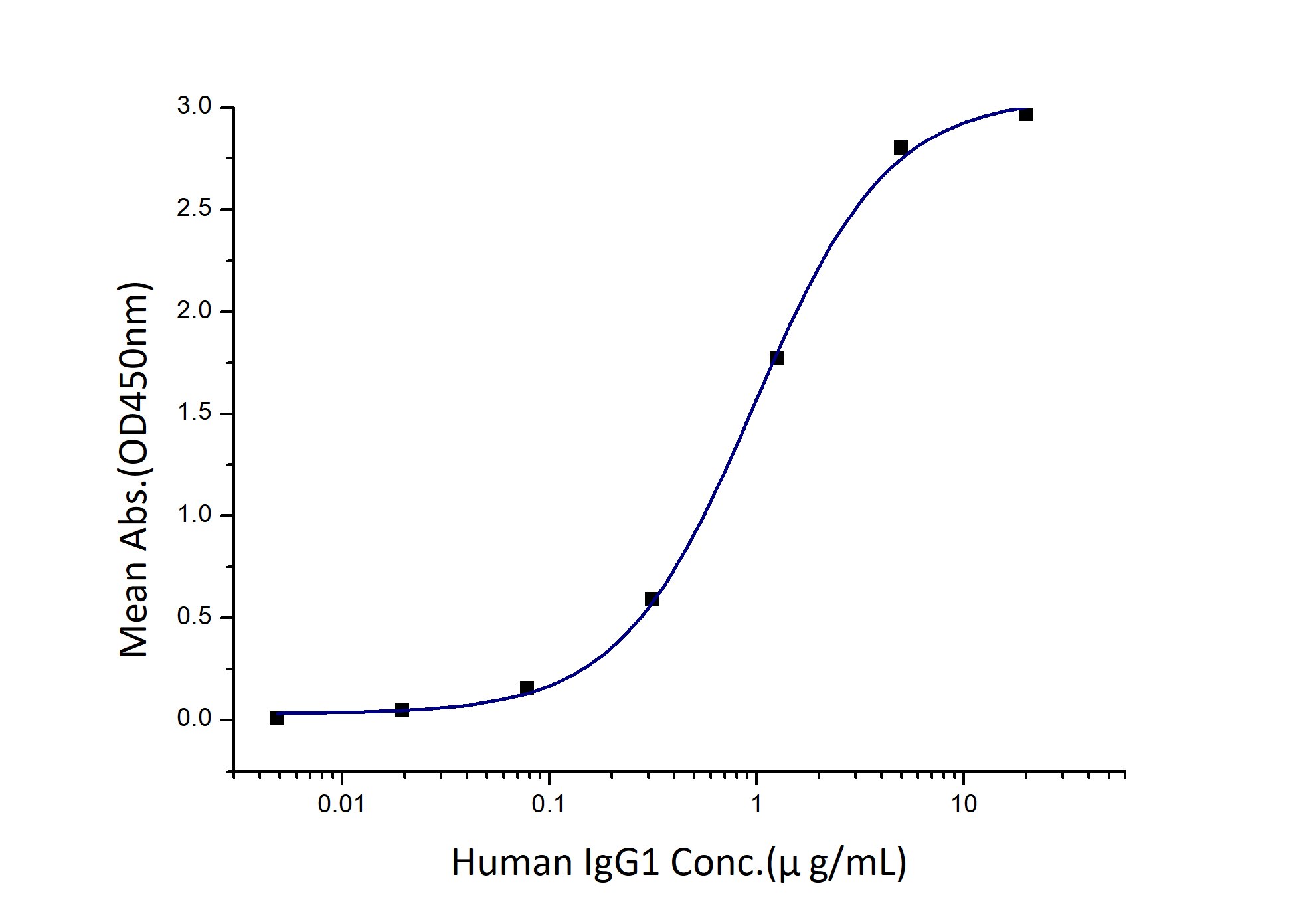
Activity
EC50: 0.5-2 μg/mL
Cat no : Eg31662
Validation Data Gallery
Product Information
| Purity | >90 %, SDS-PAGE |
| Endotoxin | <0.1 EU/μg protein, LAL method |
| Activity |
Immobilized Human FCGR3A (F176V) (Myc tag, His tag) at 2 μg/mL (100 μL/well) can bind Human IgG1 with a linear range of 0.5-2 μg/mL. |
| Expression | HEK293-derived Human FCGR3A protein Gly17-Gln208 (Accession# P08637, F176V) with a Myc tag and a His tag at the C-terminus. |
| GeneID | 2214 |
| Accession | P08637 |
| PredictedSize | 24.3 kDa |
| SDS-PAGE | 38-60 kDa, reducing (R) conditions |
| Formulation | Lyophilized from 0.22 μm filtered solution in PBS, pH 7.4. Normally 5% trehalose and 5% mannitol are added as protectants before lyophilization. |
| Reconstitution | Briefly centrifuge the tube before opening. Reconstitute at 0.1-0.5 mg/mL in sterile water. |
| Storage Conditions |
It is recommended that the protein be aliquoted for optimal storage. Avoid repeated freeze-thaw cycles.
|
| Shipping | The product is shipped at ambient temperature. Upon receipt, store it immediately at the recommended temperature. |
Background
CD16, also known as the low affinity Fc gamma receptor III for IgG (FcγRIII), exists in two isoforms, FcγRIIIa (CD16a) and FcγRIIIb (CD16b), encoded by two nearly identical genes, FCGR3A and the FCGR3B. CD16a is expressed on NK cells, macrophages, and placental trophoblasts as a polypeptide-anchored transmembrane protein while CD16b is expressed on neutrophils in a glycosylphosphatidylinositol (GPI)-anchored form. CD16a forms a heteromeric structure with the Fc epsilon RI (gamma) and/or CD3 (zeta) subunits. CD16 mediates antibody-dependent cellular cytotoxicity (ADCC) and other antibody-dependent responses, such as phagocytosis.
References:
1. S Nagarajan, et al. (1995) J Biol Chem. 270(43):25762-70. 2. J E Gessner, et al. (1995) J Biol Chem. 270(3):1350-61.