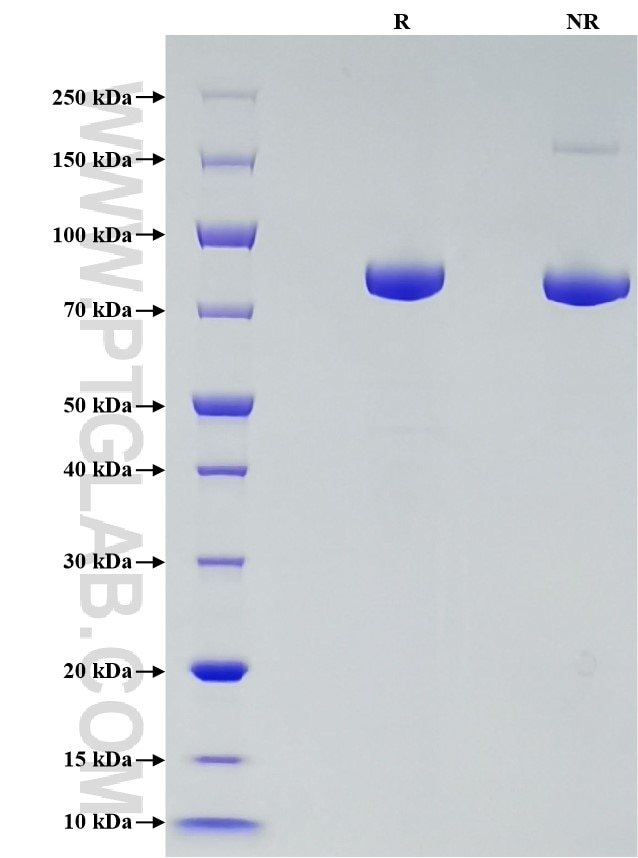
Recombinant Human Transferrin R/CD71 protein (His Tag)
Species
Human
Purity
>95 %, SDS-PAGE
Tag
His Tag
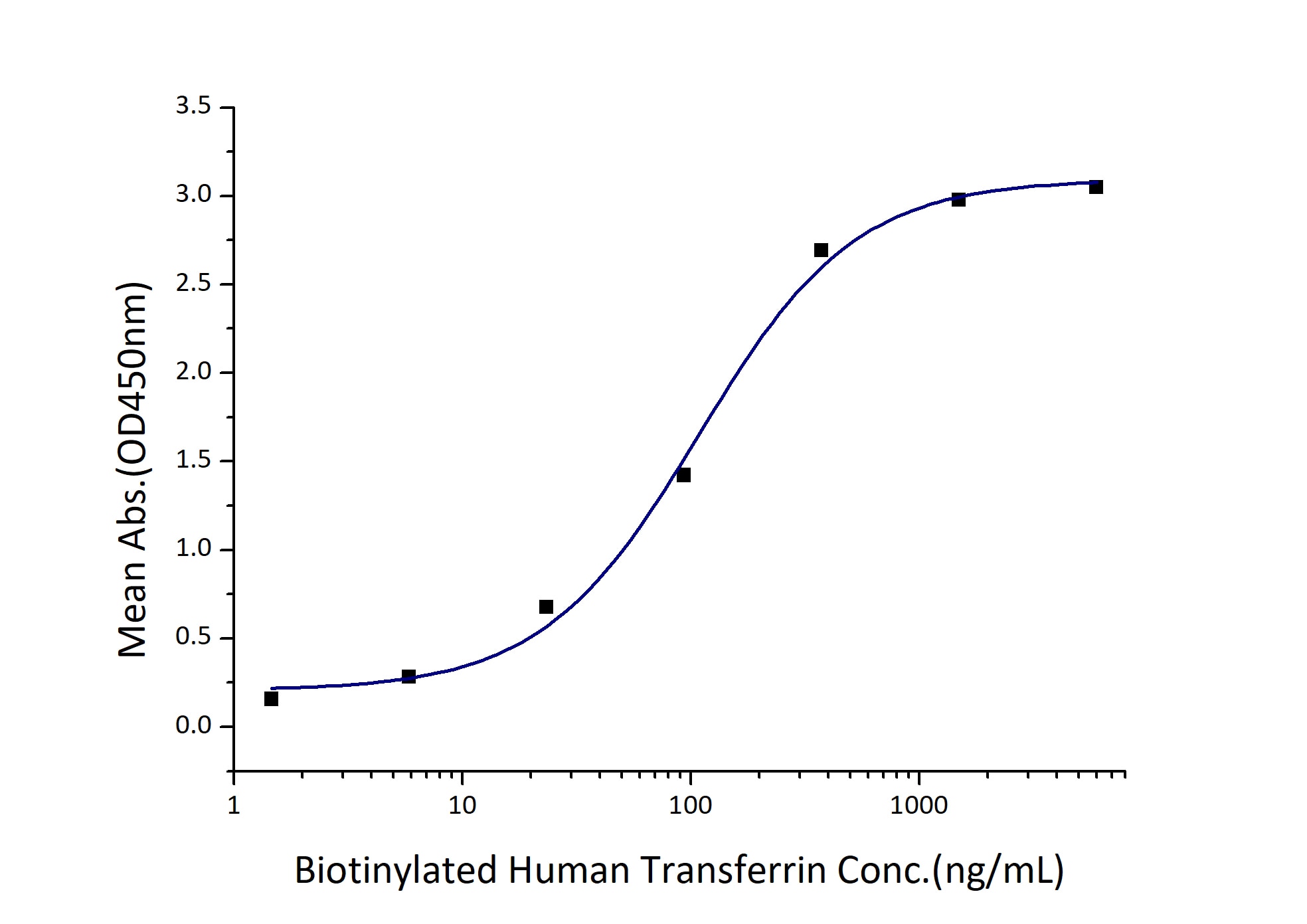
Activity
EC50: 54-216 ng/mL
Cat no : Eg0339
Validation Data Gallery
Product Information
| Purity | >95 %, SDS-PAGE |
| Endotoxin | <0.1 EU/μg protein, LAL method |
| Activity |
Immobilized Human Transferrin R (His tag) at 0.5 μg/mL (100 μL/well) can bind Biotinylated Human Transferrin (Myc tag, His tag) with a linear range of 54-216 ng/mL. |
| Expression | HEK293-derived Human Transferrin R protein Cys89-Phe760 (Accession# CAA25527) with a His tag at the N-terminus. |
| GeneID | 7037 |
| Accession | CAA25527 |
| PredictedSize | 76 kDa |
| SDS-PAGE | 75-90 kDa, reducing (R) conditions |
| Formulation | Lyophilized from 0.22 μm filtered solution in PBS, pH 7.4. Normally 5% trehalose and 5% mannitol are added as protectants before lyophilization. |
| Reconstitution | Briefly centrifuge the tube before opening. Reconstitute at 0.1-0.5 mg/mL in sterile water. |
| Storage Conditions |
It is recommended that the protein be aliquoted for optimal storage. Avoid repeated freeze-thaw cycles.
|
| Shipping | The product is shipped at ambient temperature. Upon receipt, store it immediately at the recommended temperature. |
Background
CD71 also known as transferrin receptor protein 1 (TfR1), is a transmembrane glycoprotein composed of two disulfide-linked monomers. Each monomer binds one holo-transferrin molecule creating an iron-Tf-TfR complex that enters the cell by endocytosis. CD71 is almost ubiquitously expressed, with the highest expression levels on some cells and tissues, including immature erythroid cells, placental tissue, and rapidly dividing cells. CD71 is involved in iron (Fe3+) uptake, and expression is regulated by the metabolic demand for iron. CD71 is present in actively proliferating cells and is essential for iron transport into proliferating cells.
References:
1. Speeckaert MM. et al. (2010). Crit Rev Clin Lab Sci. 47(5-6):213-228. 2. Dong HY. et al. (2011). Am J Surg Pathol. 35(5):723-732. 3. Jabara HH. et al. (2016). Nat Genet. 48(1):74-78. 4. Senyilmaz D. et al. (2015). Nature. 525(7567):124-128.