Atrial and ventricular myocytes
A guide to atrial and ventricular myocyte markers
Introduction
Human cardiomyocytes (CMs) from human pluripotent stem cells (hPSCs) have become an important source for cardiac regeneration. They are of significant interest in cardiac disease drug-related studies (1). The majority are characterized by spontaneous beating, displaying a broad range of depolarization-repolarization patterns, suggesting mixed populations of atrial and ventricular myocytes. Atrial and ventricular cardiomyocytes form the muscular walls of the heart (the myocardium). Atrial myocytes have a different ultrastructure compared to ventricular myocytes. They have differential gene expression patterns regarding, e.g., transcription factors, structural proteins, and ion channels (2). They also display distinct functions (Table 1). The highly homogeneous atrial- and ventricular-like myocytes are extremely valuable for drug safety, cardiac evaluation, and cell therapies for heart infarction.
Table 1. Differential gene expression patterns displayed in atrial and ventricular cardiomyocytes.
| Gene | Chamber | Functions |
| ANF/HESX1 | Atria | Control blood pressure, renal function, salt balance |
| HRT1/HEY1 | Atria | Transcriptional repressor of GATA4/6, ANF, BMP2, TBX2; Precise formation of the atrioventricular boundary |
| MYL7/MLC-2a | Atria | Form cardiac sarcomere; Maintenance of atrial contractility |
| Sarcolipin/SLN | Atria | Regulate calcium cycling (decrease SR calcium transport by inhibiting SERCA) |
| HRT2/HEY2 | Ventricles | Transcriptional repressor of GATA4/6, ANF, BMP2, TBX2, CX40, TBX5, ALC-1, MLC-2a; Formation of the atrioventricular canal; Maintain normal contractility of the ventricles |
| KCNE1 | Ventricles | Accessory (beta) subunit of the pore-forming (alfa) KCNQ1 that encodes the Iks |
| MYL2/MLC-2v | Ventricles | Form cardiac sarcomere; Maintenance of ventricular contractility |
Myosin light chain 2-based selection of atrial and ventricular cardiomyocytes
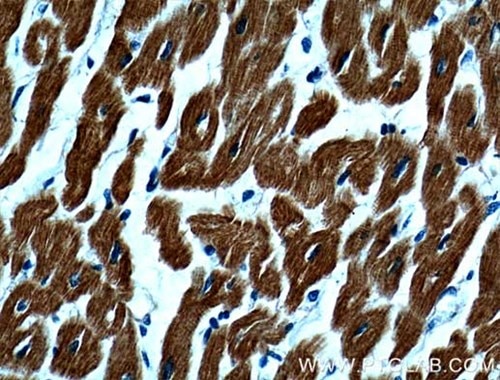
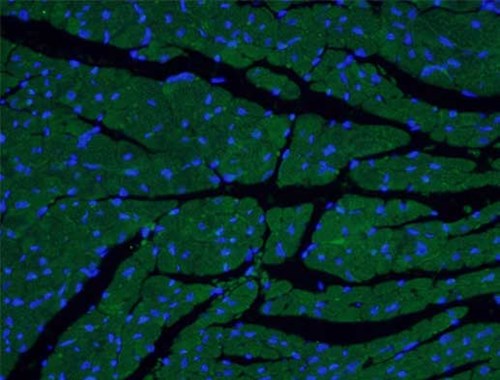
The ventricular myosin light chain-2 isoform (MYL2/MLC-2v, Figure 1) is restricted to the ventricular segment of the heart and is related to the ventricles throughout its development. In contrast, the atrial myosin light chain-2 (MYL7/MLC-2a, Figure 2) is expressed in the presumptive ventricle prior to MYL2/MLC-2v (3). Its ventricular expression is subsequently down-regulated instead, with abundant expression in the atrium postnatally. The expression pattern of MYL2/MLC-2v and MYL7/MLC-2a is considered as a specific marker for ventricle and atrial cardiomyocytes, commonly used for in vitro development of induced pluripotent stem cell-derived cardiomyocytes (iPSC-Derived Cardiomyocytes).
 |
 |
|
Figure 1. IHC results of paraffin-embedded human heart tissue using MYL2 Antibody (10906-1-AP; 1:200, 40x). |
Figure 2. IF analysis of fixed mouse heart tissue using MYL7 Antibody (17283-1-AP; 1:50, 40x). |
Cardiomyocyte remodeling and novel treatment strategies
Patients who suffer from cardiac failure by myocardial infarction have deteriorated heart function and a significant loss of ventricular cardiomyocytes. For this reason, a large number of mature ventricular myocytes are needed for the treatment of myocardial infarction. Therefore, while there are available and effective therapies for commonly occurring disorders of atrial cardiomyocytes such as atrial fibrillation, loss of ventricular cardiomyocytes resulting from myocardial infarction cannot be effectively treated. Hence, there is a high demand for a source of ventricular cardiomyocytes. Recent studies show that the activation of Notch signaling in zebrafish atria in response to ventricle ablation is crucial for the reprogramming of atrial cardiomyocyte into ventricular cardiomyocytes. This study shows that zebrafish heart regeneration can be achieved by transdifferentiation of differentiated atrial cardiomyocytes into ventricular cardiomyocytes (4).
A proper understanding of the molecular and functional differences between atrial and ventricular cardiomyocytes helps to define new strategies not only for embryonic stem cells (ESCs) applications but also to provide cellular models for the study of genetic atrial- or ventricular-related diseases (5). The programmed differentiation of atrial- and ventricular-like myocytes may be used to develop safe cell sources for individual treatment and personalized cardiac repair.
References
4) In vivo cardiac reprogramming contributes to zebrafish heart regeneration.
5) The human adult cardiomyocyte phenotype.