How to overcome the limitations of antibody-based affinity resins for protein purification
Spot-Cap for highly effective one-step protein purification
Introduction
The purification of peptide-tagged proteins using affinity resins is an established strategy to recover recombinant proteins. Applied peptide tags, also called epitope tags, and affinity resins lead to various levels of protein purity and yield, which also depend on the purified protein.
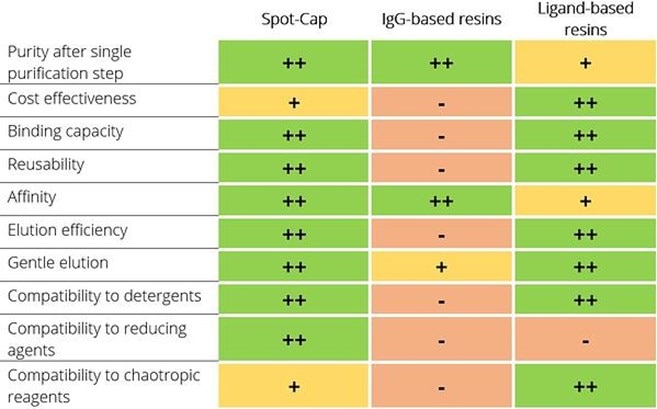
Can the advantages of an IgG resin, i.e. its high selectivity, be combined with the benefits of a chemical ligand-based resin, i.e. its high binding capacity, broad buffer stability, and easy elution? Here we discuss the features of the recently developed resin Spot-CapTM for purification of Spot-tagged proteins.
Spot-Cap
Spot-Cap is a Nanobody that binds to the Spot-Tag® and that is conjugated to agarose beads. A Nanobody or VHH is the binding domain of a heavy chain antibody from Camelids. It is also called single domain antibody. Spot-Cap in fact combines the advantages of both antibody-and chemical ligand-based resins for the purification of Spot-tagged proteins.
Spot-Tag
Spot-Tag (sequence PDRVRAVSHWSS) is a flexible, 12 amino acid peptide of 1.4 kDa size. It preserves native protein folding and full function of the Spot-tagged fusion protein because of its flexible structure. Spot-tag can be fused to the N- or C-terminus, also internal positions can be used, e.g. for transmembrane proteins to enable full flexibility during cloning and expression.
Furthermore, tag removal may not be necessary after purification because the small, inert tag is compatible with biochemical downstream assays. Spot-tag can be used to purify a wide variety of protein types including secreted, metallo-, membrane, low abundant, and sensitive proteins plus protein complexes with multiple subunits.
Advantages of Spot-Cap compared to IgG-based resins for protein purification
High binding capacity:
A high binding capacity is needed to purify recombinant proteins in a cost-effective manner. Common IgG-based resins generally provide a small binding capacity. However, Spot-Cap has a binding capacity of >340 nmol per 1 mL settled resin (>10 mg / 1 mL settled resin), which is about 10 times higher than immunoglobulin-based purification resins.
Multiple regeneration cycles:
Besides high binding capacity also multiple regeneration cycles contribute to the cost-effective purification of recombinant proteins. In most cases regeneration is done using harsh buffer conditions, which can affect the interacting capability of immunoglobulin-based resin after regeneration. In contradiction, Spot-Cap can be regenerated at least 5 times without loss in binding capacity.
High affinity:
A high affinity is needed because effective binding is only achieved if the affinity (dissociation constant) is in a range of at least 10 times lower than the tagged protein’s concentrations. Only then low expressed proteins are captured effectively by the purification resin. The Spot-Cap resin has a high affinity to the Spot-Tag and is thus capable to bind Spot-tagged proteins at low concentrations respectively in large volumes.
Gentle elution at +4°C:
Purification at low temperature is needed to minimize impacts on the stability and structural integrity of the recombinant protein. The high affinity of antibody-based purification resins may lead to an incomplete elution, which can be further reduced at low temperature. In contrast, the elution of Spot-tagged proteins from Spot-Cap has been optimized for +4°C.
Mild, competitive elution:
Antibody-based purification resins need high concentrations of up to 2 mM of peptide or elution substance, which may interfere with downstream applications and which are tedious to remove. A common alternative to elute from antibody-based purification resins is the application of acidic conditions, e.g. pH 2, which can damage the protein of interest and hence are not suited for structural and functional assays after purification. However, Spot-Cap can be eluted with only 0.1 mM Spot-peptide and the excess of Spot-peptide can be easily removed afterwards by filtration or dialysis.
Fast one-step purification:
Antibody-based purification resins generally enable the purification of recombinant proteins in a single process step at high purity, because of the IgG-based resins’ high selectivity and high affinity. This one-step process increases the yield of the purified protein, reduces costs and minimizes the exposure of the protein to potentially problematic wash/elution buffers. Chemical ligand-based resins usually require additional purification steps due to their lower specificity. In contrast, Spot-Cap can be applied for one-step purifications because of its high selectivity to Spot-tag and minimal contamination of unwanted host cell proteins.
Detergent compatibility for membrane protein purification:
Membrane proteins are purified in the presence of high concentrations of detergent at concentrations exceeding the critical micelle concentration (CMC)) to keep them soluble during the entire purification process. In addition, a flexible position of the peptide tag (N- or C-terminal or internal, though the C-terminus may be preferred due to less interference with protein localization) has to be considered if the peptide tag is covered in the detergent micelle during solubilization and can’t access the purification resin. Antibody-based resins may not be stable in the presence of detergents, because the detergent may compromise the IgG’s complex structure that is based on hydrophobic interactions. However, the Spot-Nanobody that is conjugated in Spot-Cap is functional at high concentrations of detergent, i.e. 2% DDM, 2% NP-40, or 2% Triton X-100. Also, the Spot-Tag can be positioned on both, C- and N-terminal positions as well as internal.
Compatibility to reducing agents and chaotropic reagents:
A broad buffer compatible and chemically stable purification resin is beneficial especially when working with sensitive proteins but also when working with proteins that require special buffer conditions. Special buffer components like reducing agents or chaotropic reagents may be needed to solubilize a sensitive protein, which are often incompatible with antibody-based purification resins. Antibody-based purification resins may be sensitive to reducing reagents, because the disulfide bonds of the immunoglobulin may break and disassemble the antibody. In contrast, Spot-Cap is functional in buffers containing high salt, i.e. 1 M NaCl, chaotropic reagents, i.e. 2 M Urea, and reducing reagents, i.e. 10 mM DTT, 10 mM ß-mercaptoethanol, 10 mM TCEP.
Summary
The Spot-Cap resin consists of a novel Nanobody conjugated to agarose beads. It has been developed for high capacity and selectivity for Spot-tagged proteins. In addition, Spot-tagged proteins can be effectively eluted from Spot-Cap at 4°C with low concentrations of 0.1 mM Spot peptide. The Spot-Cap resin can be frequently regenerated and therefore makes it a cost-advantageous alternative to classical IgG antibody resins. Hence, Spot-Cap can result in high purity and high yield purifications.
For more information on the Spot-Tag click here: https://www.ptglab.com/products/chromotek-nanobody-based-reagents/about/spot-capture-detection-system/
For more information on the Spot-Cap for protein purification click here: https://www.ptglab.com/products/Spot-Cap-and-Peptide-eca-ep.htm