Intracellular Flow Cytometry Staining Protocol
Understand the different approaches to intracellular staining for flow cytometry in this "do-it-yourself" guide to fix and perm.
Flow cytometry is a powerful technique used to identify groups of cells in a heterogeneous population using antibodies to measure relevant identifying markers. The detection of extracellular proteins can be done with live cells, however different steps need to be taken to detect intracellular proteins. First, fixation must occur to lock intracellular proteins in place so they cannot diffuse away following permeabilization. Next, the cell membrane needs to be permeabilized so that the antibody can gain access to its intracellular target.
There are many different methods of fixation and permeabilization, colloquially known as “fix and perm”, including popular commercial kits which contain pre-made buffers and easy-to-follow instructions. However, fix and perm can be easily performed with widely available reagents, allowing the user to save money and tweak buffer composition according to their needs. Be aware that certain tissue samples and cell types work better with certain reagents, and that some intracellular and extracellular antigens may require different methods to gain the best results. Thus, it is recommended to follow protocols or suggestions by the antibody manufacturer.
Before performing fixation and permeabilization steps, first prepare samples (from tissue homogenate or a cell line) to generate a single cell suspension, see our complete guide to flow cytometry. The fix, perm and staining steps can be done in either individual flow tubes, or, alternatively, fix and perm can be done in large batches before aliquoting for subsequent staining. The most common fixative used is 4% paraformaldehyde (PFA), other common reagents include organic solvents methanol, acetone or ethanol. In some cases, zinc based fixatives are used as these can preserve some epitopes destroyed by other fixatives (e.g. PMID:24735454). The fixation step should ideally be performed with ice-cold solutions (the fixation process is exothermic) and to gently vortex your sample to ensure maximal penetration of the fixative to all the cells (avoids cell clumping). Make sure to thoroughly wash out the fixative following the fixation steps, as this helps to avoid excessive cross-linking of proteins (increasing autofluorescence) and prevents left-over fixative cross-linking antibodies to non-specific targets.
Once fixed, cells can be permeabilized with the appropriate reagent for the sample type and desired targets. In most cases, methanol is suitable for permeabilization, and is strong enough to access cytosolic, organelle and nuclear targets. If methanol is unsuitable for your experiment requirements, a strong detergent such as Triton X can be used following crosslink fixation. Saponin is a less harsh detergent which can better preserve the native structure of certain epitopes, however it may not be strong enough to permeabilise organelle and nuclear membranes, thus reducing antibody access. Once cells are fixed and permed, subsequent antibody staining steps can then be performed.
Crosslinking fixatives such as PFA are often preferable in the study of intracellular signalling as they preserve post-translational modifications such as phosphorylation, in comparison to alcohol fixation which can cause poor detection for these targets. However, some targets such as phospho-STAT proteins are still not detectable following a traditional aldehyde-detergent fix and perm. In these cases, ice cold methanol can also be used to “unmask” such epitopes (see PMID: 16080188). It is recommended to initially follow the manufacture guidelines for which fixative and permeabilization reagents works best for that antibody and its target, as the most suitable combination will have been determined during the antibody validation process. When choosing antibodies for flow, look for manufactures which clearly state what fixative and permeabilization reagents were used for in-house validations. At Proteintech, we highlight which specific reagents we used in our product specific protocols.
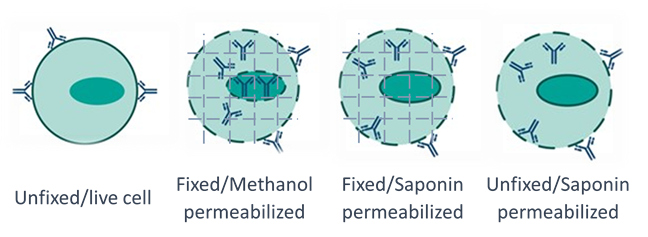
Figure 1. Schematic representation of different fix and perm approaches for antibodies to access intracellular targets.
Staining intracellular and extracellular targets
Often to answer a specific research question, cell-surface and cytoplasmic proteins need to be stained simultaneously in a flow antibody panel. This requires careful planning as there might not be a single combination of reagents that works with each member in a panel of antibodies. For example, cell phenotyping CD-marker antibodies have been developed for use on live, unfixed cells, and some of these do not work well following fix and perm protocols. Check the manufacturer recommendations prior to using cell-surface markers in a fix and perm protocol with intracellular antibodies. If this information is not available, run a trial experiment to check the antibodies’ suitability for the proposed fix and perm conditions. To do this, set up two tubes of aliquots of live cells; one which is put through the proposed fix and perm protocol prior to cell-surface marker antibody incubation, and one which goes straight to incubating the live cells with the cell-surface marker antibody with no fix and perm. If in the subsequent cytometry analysis there is a clear distinction in the positive and negative populations in the fixed and permeabilized aliquot, then the main experiment of intra- and extracellular marker antibodies can be incubated together.
If the extracellular surface marker does not work following fix and perm, then it is worth trying a different permeabilization reagent such as saponin instead of methanol. Alternatively, sequential staining steps can be performed whereby first live cells are used to incubate cell-surface marker antibodies, followed by subsequent fix and perm and intracellular antibody staining steps. Using a sequential step protocol can often lead to the best results, however it is important to check that the fluorophores for the cell surface marker antibodies are compatible with the permeabilization reagent. Protein-based fluorophores such as APC and PE are denatured following methanol permeabilization, thus inhibiting their fluorescent properties.
Fix and perm protocols
The following are example follow protocols for DIY and fix and perm, exact details will differ based on sample type and available equipment:
1. 4% PFA fixation protocol
Prepare desired sample as a single cell suspension contain 1x106 cells per individual test tubes. If required, perform cell-surface antigen staining steps prior to fixation.
1.1 Wash cells in x1 PBS and pellet cells by centrifugation (typically, ~2-5 mins at 200-300g is sufficient). Discard supernatant.
1.2 Add 4% PFA (ice cold), ~100 µl per tube, gently vortex to ensure pellet is separated. Incubate for 20 mins at room temperature.
1.3 Add x1 PBS and pellet cells (as per step 1.1). Remove supernatant into PFA waste container.
1.4 Move onto appropriate permeabilization step or resuspend in x1 PBS to store overnight at 4°C.
2. Methanol permeabilization protocol
Be aware that methanol denatures protein-based fluorophores. Do not add these prior to permeabilization.
2.1 Remove PBS (if required following overnight storage) and add ~100 µl 90% ice cold methanol and gently vortex. Cell sample must be pre-chilled.
2.2 Incubate for 15 minutes on ice.
2.3 Wash in x1 PBS to remove ethanol (as per step 1.1).
2.4 Proceed with subsequent immunostaining steps as per standard protocol.
3. Triton X-100 permeabilization protocol
Prepare the required cell permeabilization buffer to contain 0.1-0.3% Triton™ X-100 in your desired antibody buffer. Example antibody buffer is 0.5% BSA in 1xPBS.
3.1 Proceed from step 1.3 following fixation.
3.2 Resuspend in ~100 µl of cell-permeabilization buffer.
3.3 Incubate for 10 mins at room temperature.
3.4 Wash in x1 PBS to remove permeabilization buffer (as per step 1.1).
3.5 Proceed with subsequent immunostaining steps as per standard protocol.
4. Saponin permeabilization protocol
Some antigens are not compatible with stronger permeabilization agents such as methanol or Triton X-100. In these cases, the milder detergent saponin can be used. Saponin permeabilization is reversible, ensure to include saponin in subsequent wash and antibody incubation buffers following permeabilization. Prepare the cell permeabilization/wash buffer as 0.1% saponin and 0.5% BSA in x1 PBS.
4.1 Proceed from step 1.3 following fixation.
4.2 Resuspend in ~100 µl of cell-permeabilization buffer.
4.3 Incubate for 10 mins at room temperature.
4.4 Proceed with subsequent immunostaining steps, using the perm/wash buffer as above
5. Unfixed saponin permeabilization protocol
In some cases, fixatives such as PFA can denature intra-cellular antigens. In these cases, if light scatter measurement is not critical (for example in cell lines), unfixed cells can be stained for intracellular antigens in the presence of saponin. This is also a good method for the measurement of DNA content using e.g. propidium iodide.
5.1 Wash and pellet single cell suspension of 1x106 cells per tube with 1x PBS.
5.2 Add 1 ml of 0.3% saponin in 1x PBS and conjugated primary antibody to cell pellet.
5.3 Mix gently and incubate for 30 minutes at 4°C.
5.4 Spin down and discard supernatant, wash with 0.1% saponin and PBS and spin down again.
5.5 Optional: add 1-2 ml of 0.3% saponin in x1PBS with 10 µg/ml of propidium iodide. Incubate for 10 mins at 4°C.
5.6 Analyse on flow cytometer as soon as possible.
Summary
Below is a table summarising the different usages and tips for common fix and perm methods.
|
|
Methanol |
Triton-X |
Saponin |
Unfixed saponin |
|
Uses: |
Access most intracellular targets. Can be used as a standalone fix&perm. Good for DNA analysis. Good for phospho markers. |
Access most intracellular targets. Use after ice-cold PFA fixation. Good for protein based fluorophores. |
Access most intracellular targets. Not always suitable for nuclear stains. Good alternative if e.g. methanol damages target epitope. Use after ice-cold PFA fixation. |
Alternative if fixation denatures intracellular antigens. Good when measuring DNA content. |
|
Tips: |
Denatures protein based fluorophores. Make sure methanol is ice cold. |
Different perm mechanism may be better for certain clones/targets. |
Saponin perm is reversible. Use in all subsequent buffers during protocol. |
Can affect light scatter measurements. |
Further resources
For further resources, please see this highly cited paper on the comparison of different fixation and permeabilization techniques for phospho-protein targets by Krutzik and Nolan, 2003 Cytometry Part A (PMID: 14505311). If you are looking for more information on general flow cytometry, please see this detailed review by Cossarizza et al. 2019 Eur J Immunology (PMID: 31633216).
Related Content
Understanding the different viability dyes available for flow cytometry
Go with the Flow - Make the most of this powerful technique
Using Flow Cytometry with Epigenetics
Support
Newsletter Signup
Stay up-to-date with our latest news and events. New to Proteintech? Get 10% off your first order when you sign up.
