Recombinant Human IFN-gamma protein (Myc Tag, His Tag)
Species
Human
Purity
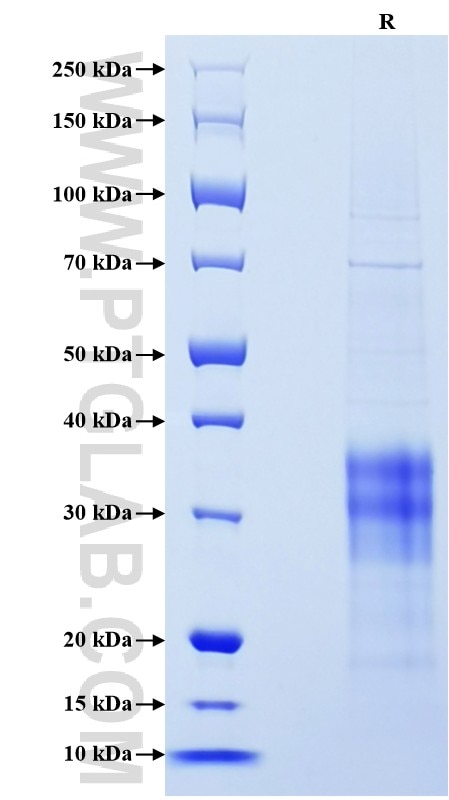
>90 %, SDS-PAGE
Tag
Myc Tag, His Tag
Activity
not tested
Cat no : Eg0004
Validation Data Gallery
Product Information
| Purity | >90 %, SDS-PAGE |
| Endotoxin | <0.1 EU/μg protein, LAL method |
| Activity |
Not tested |
| Expression | HEK293-derived Human IFN-gamma protein Gln24-Gly161 (Accession# P01579) with a Myc tag and a His tag at the C-terminus. |
| GeneID | 3458 |
| Accession | P01579 |
| PredictedSize | 21.2 KDa |
| SDS-PAGE | 25-38 kDa, reducing (R) conditions |
| Formulation | Lyophilized from 0.22 μm filtered solution in PBS, pH 7.4. Normally 5% trehalose and 5% mannitol are added as protectants before lyophilization. |
| Reconstitution | Briefly centrifuge the tube before opening. Reconstitute at 0.1-0.5 mg/mL in sterile water. |
| Storage Conditions |
It is recommended that the protein be aliquoted for optimal storage. Avoid repeated freeze-thaw cycles.
|
| Shipping | The product is shipped at ambient temperature. Upon receipt, store it immediately at the recommended temperature. |
Background
Interferon gamma (IFN γ) is a type II interferon that confers immunity to bacterial, viral and protozoan infections. In its active form, IFN γ is a glycosylated, non-covalently linked homodimer of 29-32 kDa subunits. It is produced by a number of immune cell types, including natural killer cells, natural killer T cells and effector lymphocyte T cells, in response to antigenic and inflammatory stimuli. The IFN-γ dimer binds to its cognate receptor, which has two subunits: IFN-γR1, the ligand binding chain (α chain), and IFN-γR2, the signal transducing chain (β chain). Binding to the receptor activates the JAK/STAT pathway, which in turn activates IFN-γ responsive genes. While IFN γ can inhibit viral replication, it also acts as an immunomodulator and immunostimulator by increasing the surface expression of class I MHC proteins.
References:
1.Billiau, Alfons et al. Cytokine & growth factor reviews vol. 20,2 (2009): 97-113. 2.Tau, G et al. Allergy vol. 54,12 (1999): 1233-51. 3.Ding, Huihui et al. Biomedicine & pharmacotherapy = Biomedecine & pharmacotherapie vol. 155, (2022): 113683. 4.Cordeiro, Patrícia Azevedo Soares et al. Revista do Instituto de Medicina Tropical de Sao Paulo vol. 64, (2022): e64.