Tested Applications
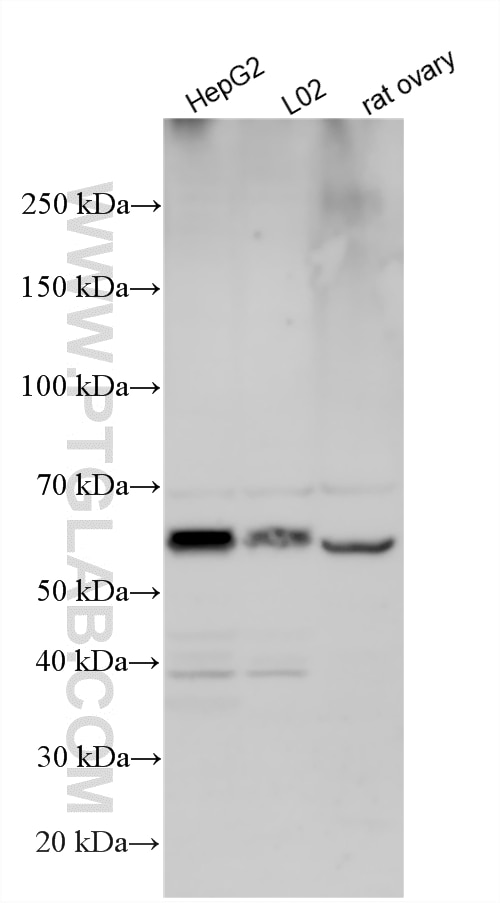
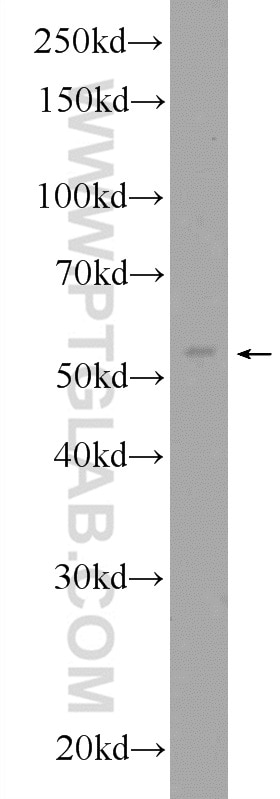
| Positive WB detected in | HepG2 cells, A431 cells, L02 cells, Rat ovary cells |
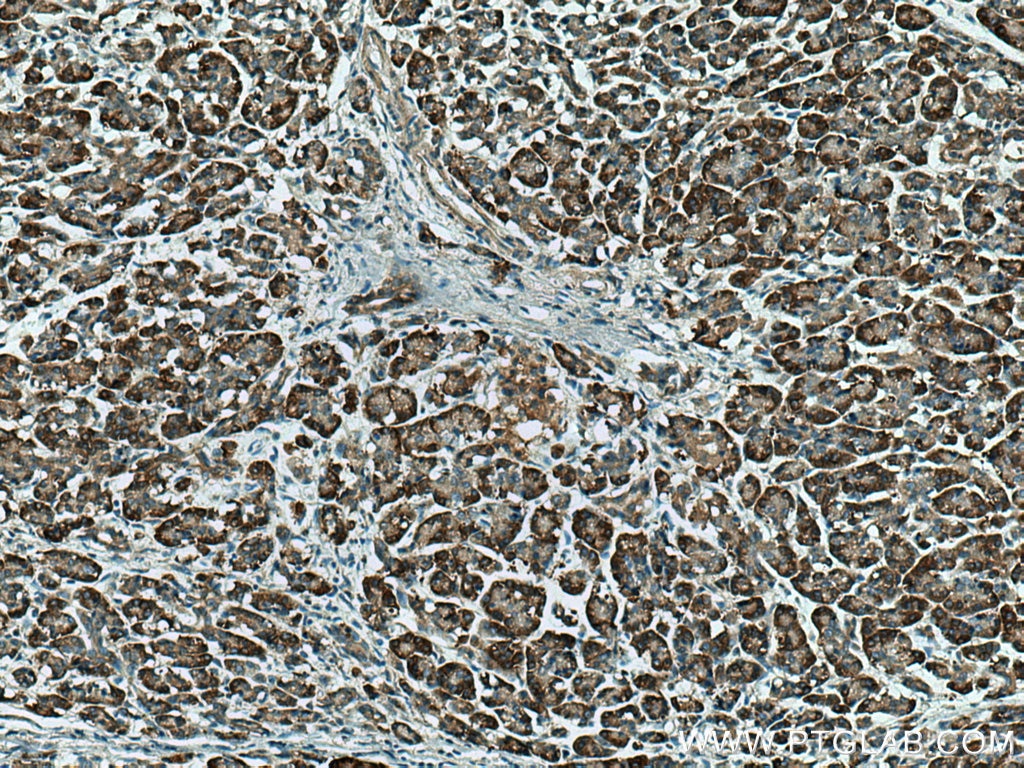
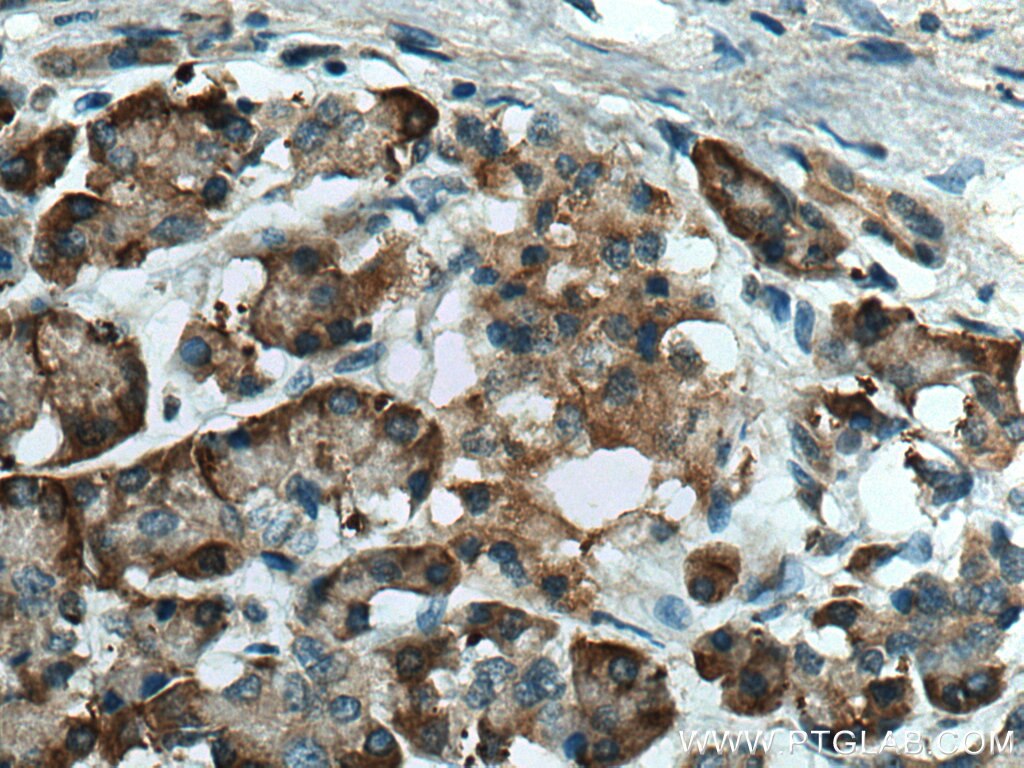
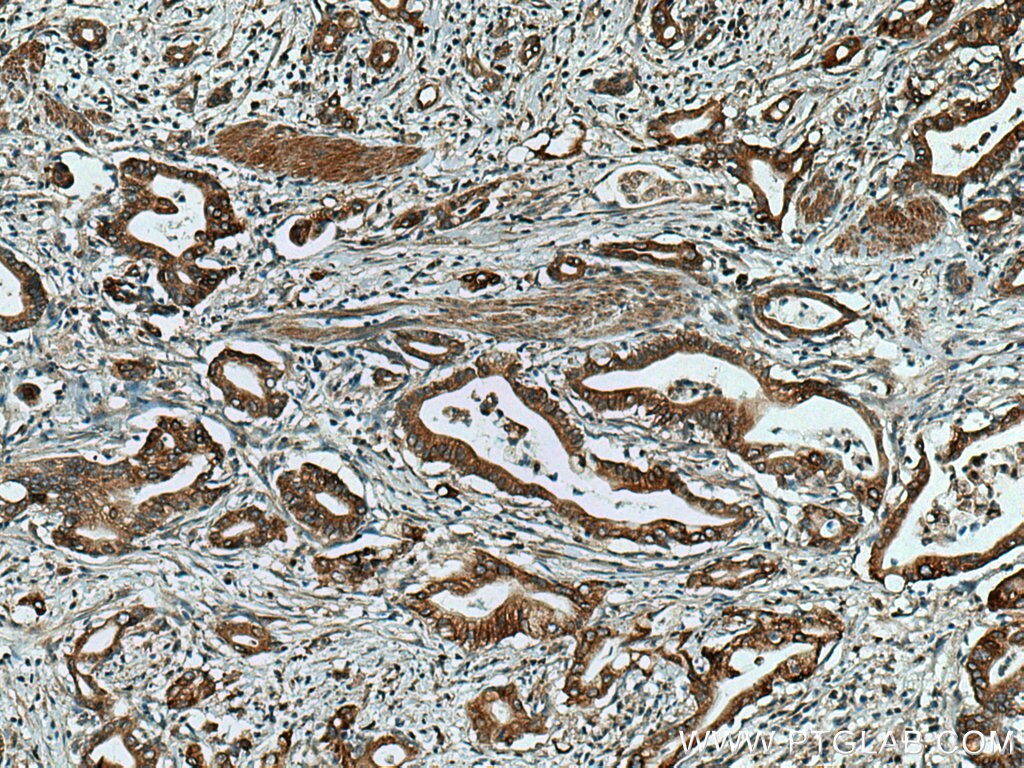
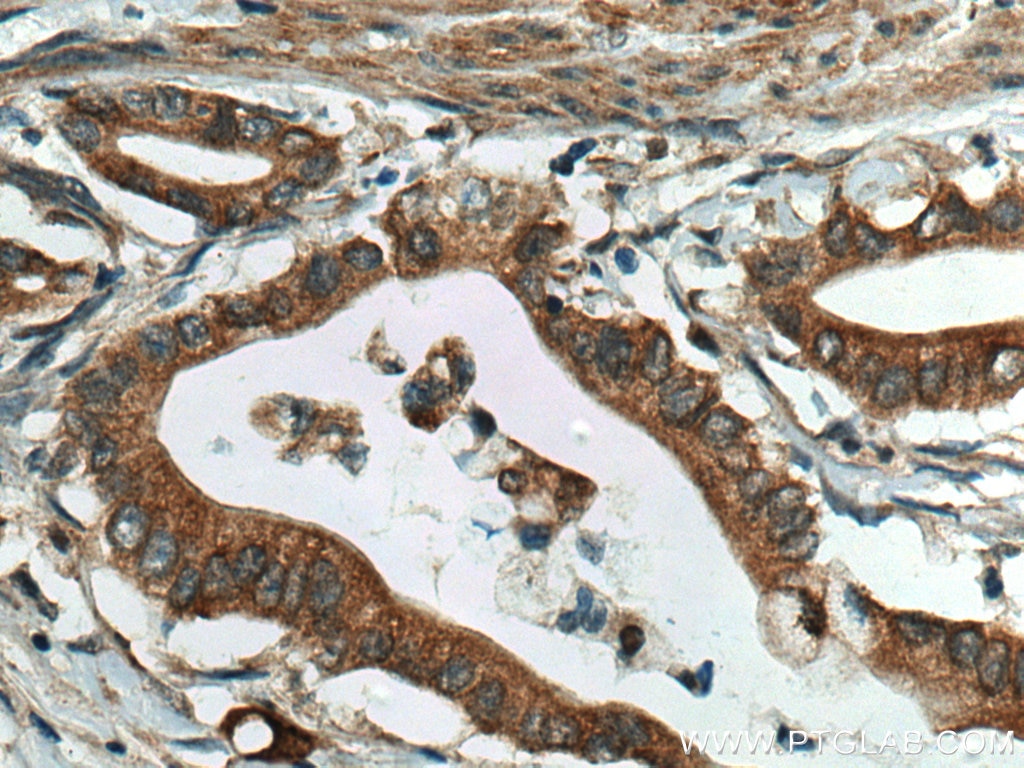
| Positive IHC detected in | human pancreas cancer tissue Note: suggested antigen retrieval with TE buffer pH 9.0; (*) Alternatively, antigen retrieval may be performed with citrate buffer pH 6.0 |
Recommended dilution
| Application | Dilution |
|---|---|
| Western Blot (WB) | WB : 1:500-1:3000 |
| Immunohistochemistry (IHC) | IHC : 1:50-1:500 |
| It is recommended that this reagent should be titrated in each testing system to obtain optimal results. | |
| Sample-dependent, Check data in validation data gallery. | |
Published Applications
| WB | See 14 publications below |
| IHC | See 1 publications below |
| IF | See 3 publications below |
| CoIP | See 1 publications below |
Product Information
12007-1-AP targets ERO1L in WB, IHC, IF, CoIP, ELISA applications and shows reactivity with human, mouse, rat samples.
| Tested Reactivity | human, mouse, rat |
| Cited Reactivity | human, mouse, rat, pig |
| Host / Isotype | Rabbit / IgG |
| Class | Polyclonal |
| Type | Antibody |
| Immunogen |
CatNo: Ag2620 Product name: Recombinant human ERO1L protein Source: e coli.-derived, PGEX-4T Tag: GST Domain: 20-390 aa of BC008674 Sequence: SGHGEEQPPETAAQRCFCQVSGYLDDCTCDVETIDRFNNYRLFPRLQKLLESDYFRYYKVNLKRPCPFWNDISQCGRRDCAVKPCQSDEVPDGIKSASYKYSEEANNLIEECEQAERLGAVDESLSEETQKAVLQWTKHDDSSDNFCEADDIQSPEAEYVDLLLNPERYTGYKGPDAWKIWNVIYEENCFKPQTIKRPLNPLASGQGTSEENTFYSWLEGLCVEKRAFYRLISGLHASINVHLSARYLLQETWLEKKWGHNITEFQQRFDGILTEGEGPRRLKNLYFLYLIELRALSKVLPFFERPDFQLFTGNKIQDEENKMLLLEILHEIKSFPLHFDENSFFAGDKKEAHKLKEDFRLHFRNISRIMD Predict reactive species |
| Full Name | ERO1-like (S. cerevisiae) |
| Calculated Molecular Weight | 468 aa, 54 kDa |
| Observed Molecular Weight | 54 kDa |
| GenBank Accession Number | BC008674 |
| Gene Symbol | ERO1L |
| Gene ID (NCBI) | 30001 |
| RRID | AB_10666441 |
| Conjugate | Unconjugated |
| Form | Liquid |
| Purification Method | Antigen affinity purification |
| UNIPROT ID | Q96HE7 |
| Storage Buffer | PBS with 0.02% sodium azide and 50% glycerol, pH 7.3. |
| Storage Conditions | Store at -20°C. Stable for one year after shipment. Aliquoting is unnecessary for -20oC storage. 20ul sizes contain 0.1% BSA. |
Background Information
ERO1L, also named as ERO1-alpha, is an essential oxidoreductase that oxidizes proteins in the endoplasmic reticulum to produce disulfide bonds. It acts by oxidizing directly P4HB/PDI isomerase through a direct disulfide exchange. It does not act as a direct oxidant of folding substrate, but relies on P4HB/PDI to transfer oxidizing equivalent. Associates with ERP44 but not with GRP54, demonstrating that it does not oxidize all PDI related proteins and can discriminate between PDI and related proteins. Its reoxidation probably involves electron transfer to molecular oxygen via FAD. Glutathione may be required to regulate its activity in the endoplasmic reticulum. It may be responsible for a significant proportion of reactive oxygen species (ROS) in the cell, thereby being a source of oxidative stress. It is required for the folding of immunoglobulin proteins. Responsible for the release of the unfolded cholera toxin from reduced P4HB/PDI in case of infection by V.cholerae, thereby playing a role in retrotranslocation of the toxin. This antibody has no cross reaction to ERO1.
Protocols
| Product Specific Protocols | |
|---|---|
| IHC protocol for ERO1L antibody 12007-1-AP | Download protocol |
| WB protocol for ERO1L antibody 12007-1-AP | Download protocol |
| Standard Protocols | |
|---|---|
| Click here to view our Standard Protocols |
Publications
| Species | Application | Title |
|---|---|---|
Cancers (Basel) ER Stress Response and Induction of Apoptosis in Malignant Pleural Mesothelioma: The Achilles Heel Targeted by the Anticancer Ruthenium Drug BOLD-100 | ||
Cell Calcium Mannan-binding lectin deficiency augments hepatic endoplasmic reticulum stress through IP3R-controlled calcium release. | ||
J Virol Porcine circovirus 2 manipulates PERK-ERO1α axis of endoplasmic reticulum in favor of its replication by derepressing viral DNA from HMGB1 sequestration within nuclei. | ||
J Toxicol Oxidative Stress and the ER Stress Response in a Murine Model for Early-Stage Alcoholic Liver Disease. | ||
Adv Sci (Weinh) Inhibition of Macrophage ARID3A Alleviates Myocardial Ischemia-Reperfusion Injury After Heart Transplantation by Reducing THBS1/CD47 Signaling-Mediated Neutrophil Extracellular Traps Formation | ||
BMC Genomics Mechanisms underlying the estradiol mediated protection of yak oviduct epithelial cells against ER-Ca2+ imbalance |