Recombinant Human BST2 protein (rFc Tag)
Species
Human
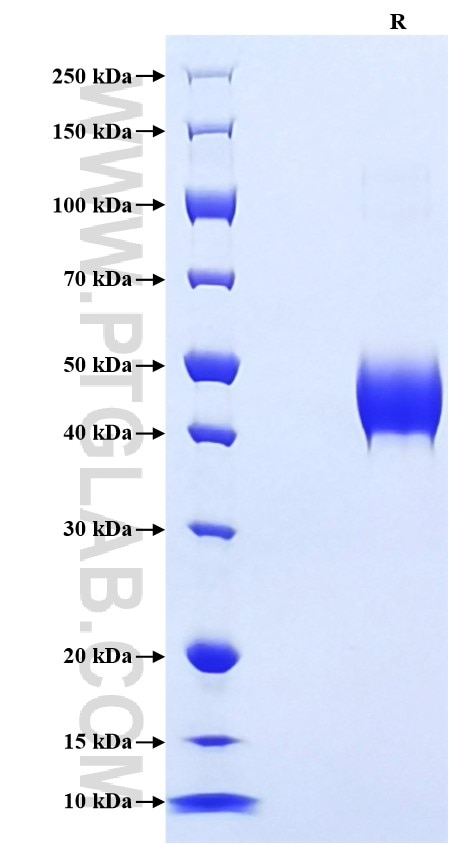
Purity
>90 %, SDS-PAGE
Tag
rFc Tag
Activity
not tested
Cat no : Eg1961
Validation Data Gallery
Product Information
| Purity | >90 %, SDS-PAGE |
| Endotoxin | <0.1 EU/μg protein, LAL method |
| Activity |
Not tested |
| Expression | HEK293-derived Human BST2 protein Asn49-Ser161 (Accession# Q10589-1) with a rabbit IgG Fc tag at the N-terminus. |
| GeneID | 684 |
| Accession | Q10589-1 |
| PredictedSize | 39.8 kDa |
| SDS-PAGE | 40-50 kDa, reducing (R) conditions |
| Formulation | Lyophilized from 0.22 μm filtered solution in PBS, pH 7.4. Normally 5% trehalose and 5% mannitol are added as protectants before lyophilization. |
| Reconstitution | Briefly centrifuge the tube before opening. Reconstitute at 0.1-0.5 mg/mL in sterile water. |
| Storage Conditions |
It is recommended that the protein be aliquoted for optimal storage. Avoid repeated freeze-thaw cycles.
|
| Shipping | The product is shipped at ambient temperature. Upon receipt, store it immediately at the recommended temperature. |
Background
BST2, also named as CD317 and Tetherin, belongs to the tetherin family. It may be involved in the sorting of secreted proteins and it is involved in pre-B-cell growth. BST2 is an antiretroviral defense protein, that blocks retrovirus release from the cell surface. Depleted upon HIV-1 infection by viral VPU protein through 20S proteasome degradation. Depleted upon infection by human Kaposi's sarcoma-associated herpesvirus (KSHV) through ubiquitination and subsequent degradation. BST2 may play a role in B-cell activation in rheumatoid arthritis. It is recently identified interferon-induced cellular proteins that restrict infections by retroviruses and filoviruses and of influenza virus and flaviviruses, respectively. BST2 is a plasma membrane protein, tetherin inhibits virion particle release from infected cells. BST2 is effective against retroviruses and flavoviruses whilst IFITMs disrupt influenza and flavivirus infection.
References:
1. Miyagi E. et al. (2009). Proc Natl Acad Sci U S A. 106(8):2868-2873. 2. Skasko M. et al. (2011). Virology. 411(1):65-77. 3. Andrew AJ. et al. (2009). Retrovirology. 6:80. 4. Tanwattana N. et al. (2023). PLoS One. 18(11):e0292833.