Recombinant Human ECM1 protein (His Tag)
Species
Human
Purity
>95 %, SDS-PAGE
Tag
His Tag
Activity
not tested
Cat no : Eg0533
Validation Data Gallery
Product Information
| Purity | >95 %, SDS-PAGE |
| Endotoxin | <0.1 EU/μg protein, LAL method |
| Activity |
Not tested |
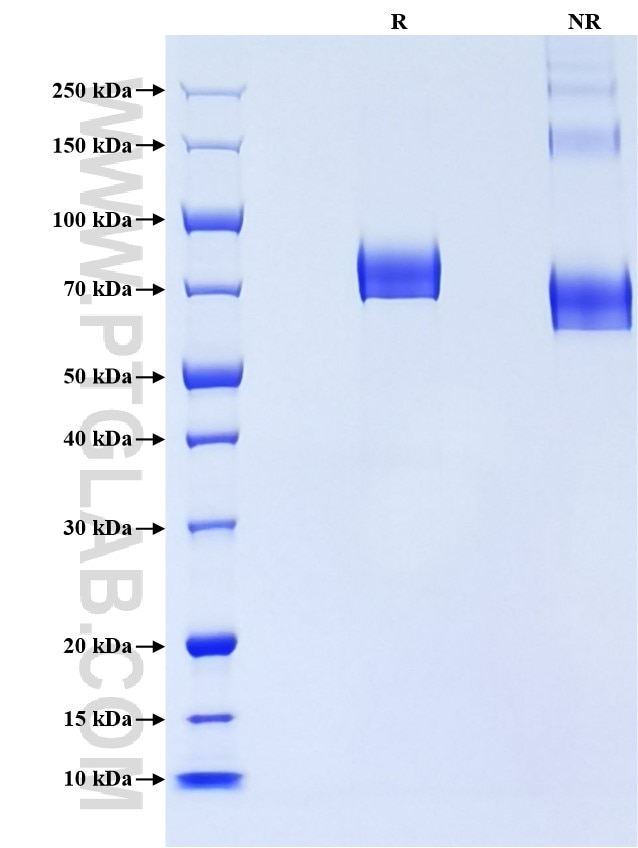
| Expression | HEK293-derived Human ECM1 protein Ala20-Glu540 (Accession# AAH23505) with a His tag at the C-terminus. |
| GeneID | 1893 |
| Accession | AAH23505 |
| PredictedSize | 63 kDa |
| SDS-PAGE | 68-90 kDa, reducing (R) conditions |
| Formulation | Lyophilized from 0.22 μm filtered solution in PBS, pH 7.4. Normally 5% trehalose and 5% mannitol are added as protectants before lyophilization. |
| Reconstitution | Briefly centrifuge the tube before opening. Reconstitute at 0.1-0.5 mg/mL in sterile water. |
| Storage Conditions |
It is recommended that the protein be aliquoted for optimal storage. Avoid repeated freeze-thaw cycles.
|
| Shipping | The product is shipped at ambient temperature. Upon receipt, store it immediately at the recommended temperature. |
Background
ECM1 (extracellular matrix protein 1), also known as URBWD. It is located in secreted, extracellular space, and extracellular matrix, which is mainly expressed in esophagus and gall bladder. The gene encodes a soluble protein that is involved in endochondral bone formation, angiogenesis, and tumor biology. It also interacts with a variety of extracellular and structural proteins, contributing to the maintenance of skin integrity and homeostasis. Mutations in this gene are associated with lipoid proteinosis disorder (also known as hyalinosis cutis et mucosae or Urbach-Wiethe disease) that is characterized by generalized thickening of skin, mucosae and certain viscera. The calculated molecular weight of ECM1 is 60 kDa, and this antibody can recognize the 63 kDa isoform of target, moreover, this protein may have glycosylation modification.
References:
1. Chen H. et al. (2010). Med Oncol. 1:S318-25. 2. López-Marure R. et al. (2011). Eur J Pharmacol. 660(2-3):268-74. 3. Lee KM. et al. (2015). Oncogene. 34(50):6055-65.