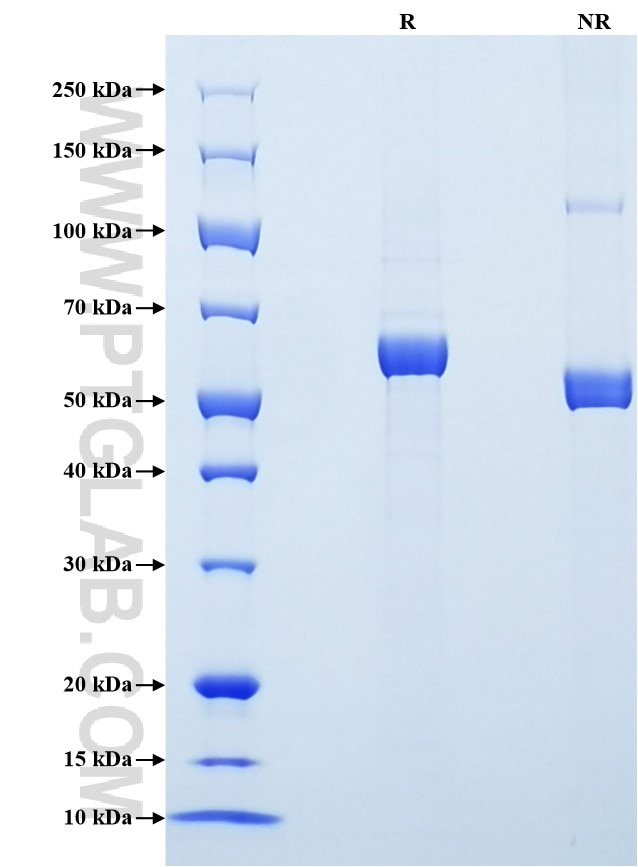
Recombinant Human MMP-3 protein (His Tag)
Species
Human
Purity
>90 %, SDS-PAGE
Tag
His Tag
Activity
not tested
Cat no : Eg0382
Validation Data Gallery
Product Information
| Purity | >90 %, SDS-PAGE |
| Endotoxin | <0.1 EU/μg protein, LAL method |
| Activity |
Not tested |
| Expression | HEK293-derived Human MMP-3 protein Tyr18-Cys477 (Accession# P08254) with a His tag at the C-terminus. |
| GeneID | 4314 |
| Accession | P08254 |
| PredictedSize | 56 kDa |
| SDS-PAGE | 55-65 kDa, reducing (R) conditions |
| Formulation | Lyophilized from 0.22 μm filtered solution in PBS, pH 7.4. Normally 5% trehalose and 5% mannitol are added as protectants before lyophilization. |
| Reconstitution | Briefly centrifuge the tube before opening. Reconstitute at 0.1-0.5 mg/mL in sterile water. |
| Storage Conditions |
It is recommended that the protein be aliquoted for optimal storage. Avoid repeated freeze-thaw cycles.
|
| Shipping | The product is shipped at ambient temperature. Upon receipt, store it immediately at the recommended temperature. |
Background
MMP-3 (matrix metallopeptidase 3), also named as Stromelysin 1 or STR1, is a member of matrix metalloproteinase (MMP) family. The MMP family of enzymes is comprised of critically important extracellular matrix remodeling proteases whose activity has been implicated in normal embryogenesis and tissue remodelling and in many diseases such as arthritis, cancer, periodontitis, glomerulonephritis, encephalomyelitis, atherosclerosis and tissue ulceration. These proteases have come to represent important therapeutic and diagnostic targets for the treatment and detection of human cancers. MMP-3 is secreted from the cells as a proenzyme. The proenzyme has been shown to stimulate plasminogen activation. The active MMP-3 is capable of cleaving types III, IV, IX and X collagen, aggrecan, fbronectin, laminin, IGFBP-3, serpins, and IL-1β.
References:
1. Roy R. et al. (2009) Journal of Clinical Oncology. 27(31):5287-5297. 2. Nagase H. et al. (1994) Contributions to Nephrology. 107:85. 3. Stamenkovic I. et al. (2003) The Journal of Pathology. 200(4):448-464. 4. Pytliak M. et al. (2012) Onkologie. 35(1-2):49-53. 5. Begoña Arza. et al. (2000). Febs Journal. 267(21):6378. 6. Fan. et al. (2011) International Journal of Molecular Medicine. 27(4).