Recombinant Mouse PD-1 protein (His Tag)
Species
Mouse
Purity
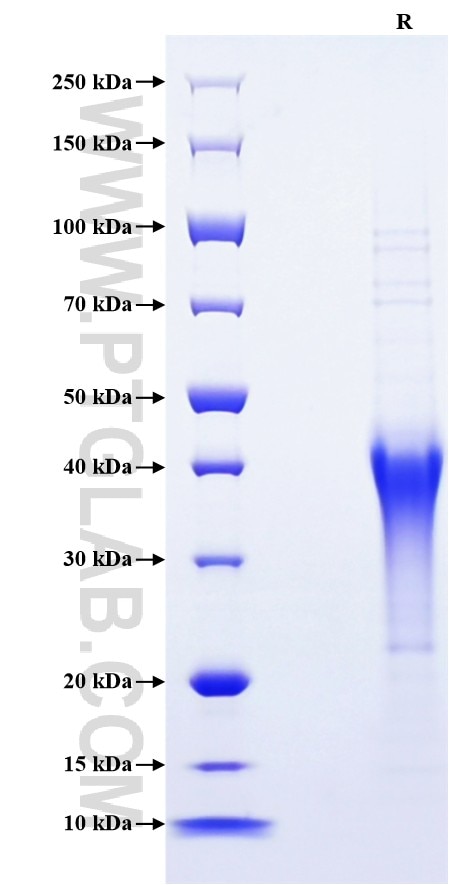
>90 %, SDS-PAGE
Tag
His Tag
Activity
not tested
Cat no : Eg0918
Validation Data Gallery
Product Information
| Purity | >90 %, SDS-PAGE |
| Endotoxin | <0.1 EU/μg protein, LAL method |
| Activity |
Not tested |
| Expression | HEK293-derived Mouse PD-1 protein Leu25-Gln167 (Accession# Q02242) with a His tag at the C-terminus. |
| GeneID | 18566 |
| Accession | Q02242 |
| PredictedSize | 17.2 kDa |
| SDS-PAGE | 35-45 kDa, reducing (R) conditions |
| Formulation | Lyophilized from 0.22 μm filtered solution in PBS, pH 7.4. Normally 5% trehalose and 5% mannitol are added as protectants before lyophilization. |
| Reconstitution | Briefly centrifuge the tube before opening. Reconstitute at 0.1-0.5 mg/mL in sterile water. |
| Storage Conditions |
It is recommended that the protein be aliquoted for optimal storage. Avoid repeated freeze-thaw cycles.
|
| Shipping | The product is shipped at ambient temperature. Upon receipt, store it immediately at the recommended temperature. |
Background
Programmed cell death 1 (PD-1, also known as CD279) is an immunoinhibitory receptor that belongs to the CD28/CTLA-4 subfamily of the Ig superfamily. It is a 288 amino acids type I transmembrane protein composed of a Ig superfamily domain, a stalk, a transmembrane domain, and an intracellular domain containing an immunoreceptor tyrosine-based inhibitory motif (ITIM), as well as an immunoreceptor tyrosine-based switch motif (ITSM). PD-1 can be expressed on activated T cells, B cells, natural killer T cells, monocytes, and dendritic cells (DCs). Engagement of PD-1 by its ligands PD-L1 or PD-L2 transduces a signal that inhibits T-cell proliferation, cytokine production, and cytolytic function. It is critical for the regulation of T cell function during tolerance, autoimmunity and infection. Blockade of PD-1 can overcome immune resistance and also has been shown to have antitumor activity.
References:
1. Arlene H Sharpe, et al. (2007) Nat Immunol. 8(3):239-45. 2. Mary E Keir, et al. (2008) Annu Rev Immunol. 26:677-704. 3. James L Riley. (2009) Immunol Rev. 229(1):114-25. 4. Loise M Francisco, et al. (2010) Immunol Rev. 236:219-42. 5. Suzanne L Topalian, et al. (2012) N Engl J Med. 366(26):2443-54.