Recombinant Mouse TNF-alpha protein (Myc Tag, His Tag)
Species
Mouse
Purity
>90 %, SDS-PAGE
Tag
Myc Tag, His Tag
Activity
not tested
Cat no : Eg31366
Validation Data Gallery
Product Information
| Purity | >90 %, SDS-PAGE |
| Endotoxin | <0.1 EU/μg protein, LAL method |
| Activity |
Not tested |
| Expression | HEK293-derived Mouse TNF-alpha protein Leu80-Leu235 (Accession# P06804) with a Myc tag and a His tag at the C-terminus. |
| GeneID | 21926 |
| Accession | P06804 |
| PredictedSize | 22.3 kDa |
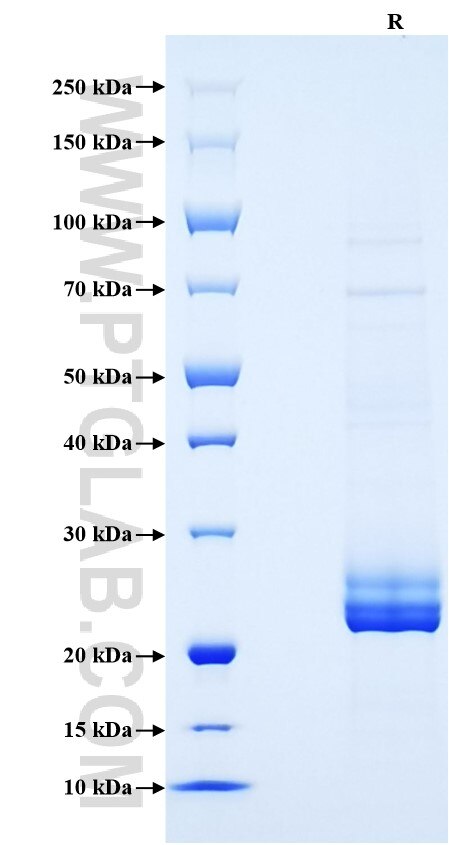
| SDS-PAGE | 22-26 kDa, reducing (R) conditions |
| Formulation | Lyophilized from 0.22 μm filtered solution in PBS, pH 7.4. Normally 5% trehalose and 5% mannitol are added as protectants before lyophilization. |
| Reconstitution | Briefly centrifuge the tube before opening. Reconstitute at 0.1-0.5 mg/mL in sterile water. |
| Storage Conditions |
It is recommended that the protein be aliquoted for optimal storage. Avoid repeated freeze-thaw cycles.
|
| Shipping | The product is shipped at ambient temperature. Upon receipt, store it immediately at the recommended temperature. |
Background
TNF, also known as TNF-alpha, or cachectin, is a multifunctional proinflammatory cytokine that belongs to the tumor necrosis factor (TNF) superfamily. It is expressed as a 26 kDa membrane bound protein and is then cleaved by TNF-alpha converting enzyme (TACE) to release the soluble 17 kDa monomer, which forms homotrimers in circulation. It is produced chiefly by activated macrophages, although it can be produced by many other cell types such as CD4+ lymphocytes, NK cells, neutrophils, mast cells, eosinophils, and neurons. It can bind to, and thus functions through its receptors TNFRSF1A/TNFR1 and TNFRSF1B/TNFBR. This cytokine is involved in the regulation of a wide spectrum of biological processes including cell proliferation, differentiation, apoptosis, lipid metabolism, and coagulation. Mouse and human TNF-alpha share 79% amino acid sequence identity. Unlike human TNF-alpha, the mouse form is glycosylated. In mouse deficiency of this gene is associated with defects in response to bacterial infection, with defects in forming organized follicular dendritic cell networks and germinal centers, and with a lack of primary B cell follicles.
References:
1. Agbanoma G. et al. (2012) J Immunol. 188: 1307-17. 2. Kriegler M. et al. (1988) Cell. 53: 45-53. 3. Theiss AL. et al. (2005) J Biol Chem. 280: 36099-109. 4. Swardfager W. et al. (2010) Biol Psychiatry. 68:930-41. 5. Locksley RM.et al. (2001) Cell. 104(4):487-501. 6. provided by RefSeq, Jun 2013