Product Information
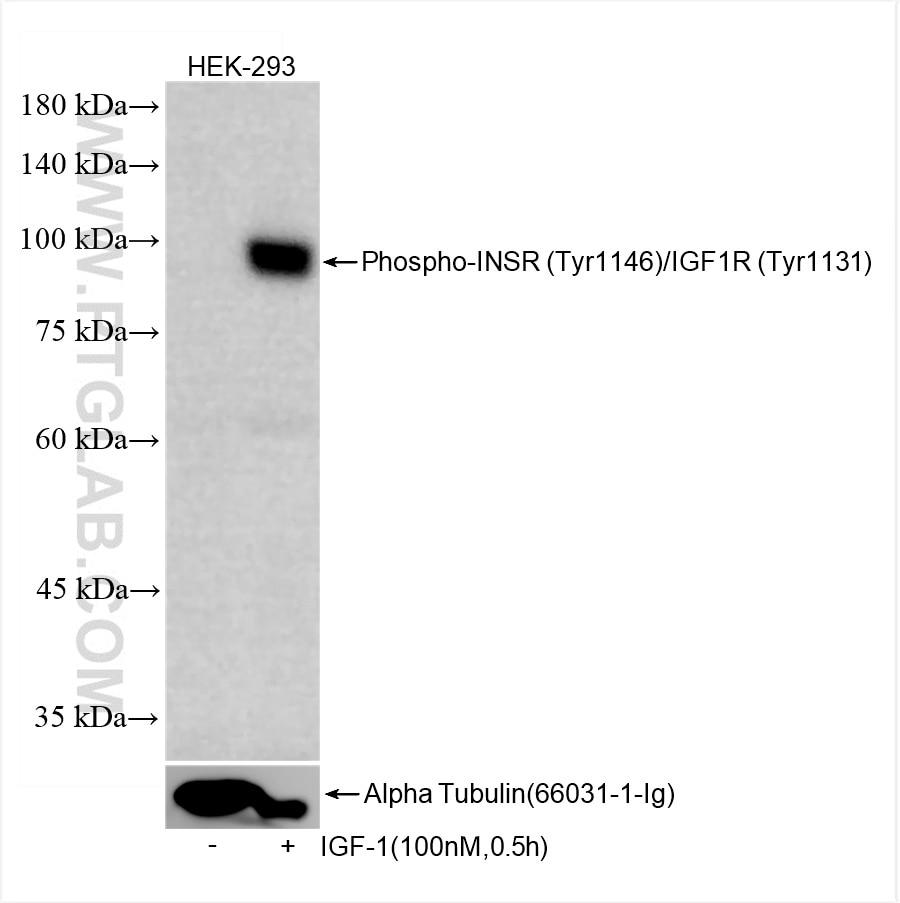
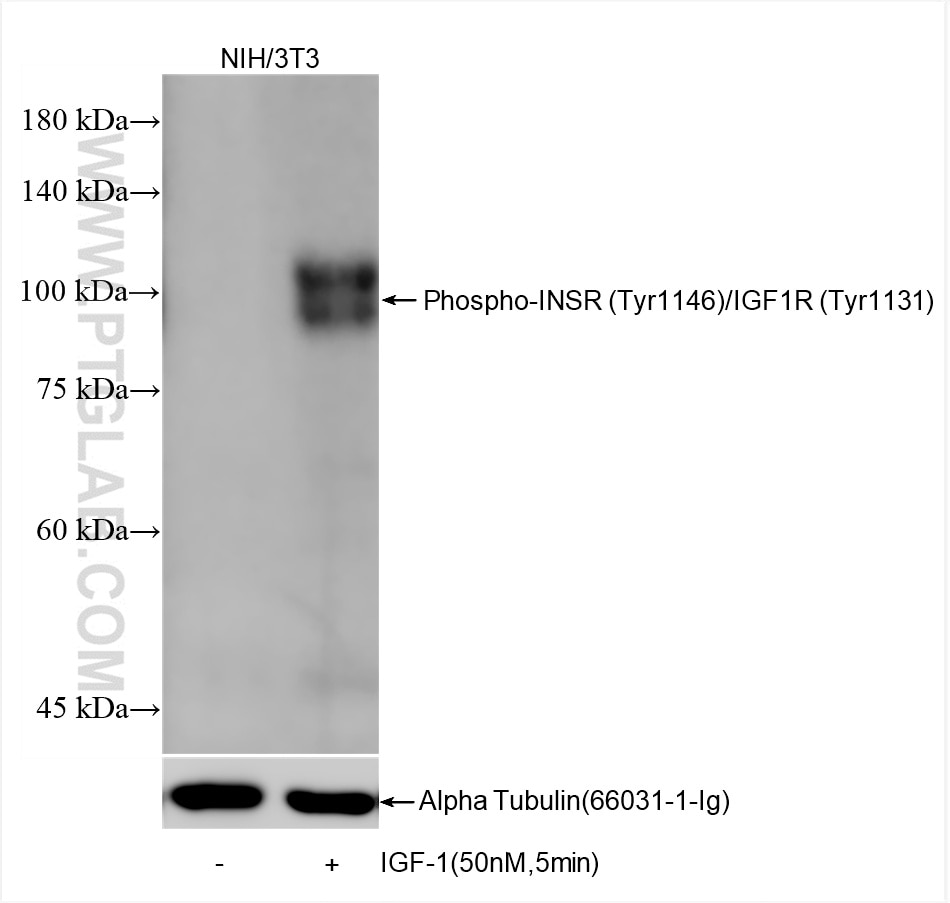
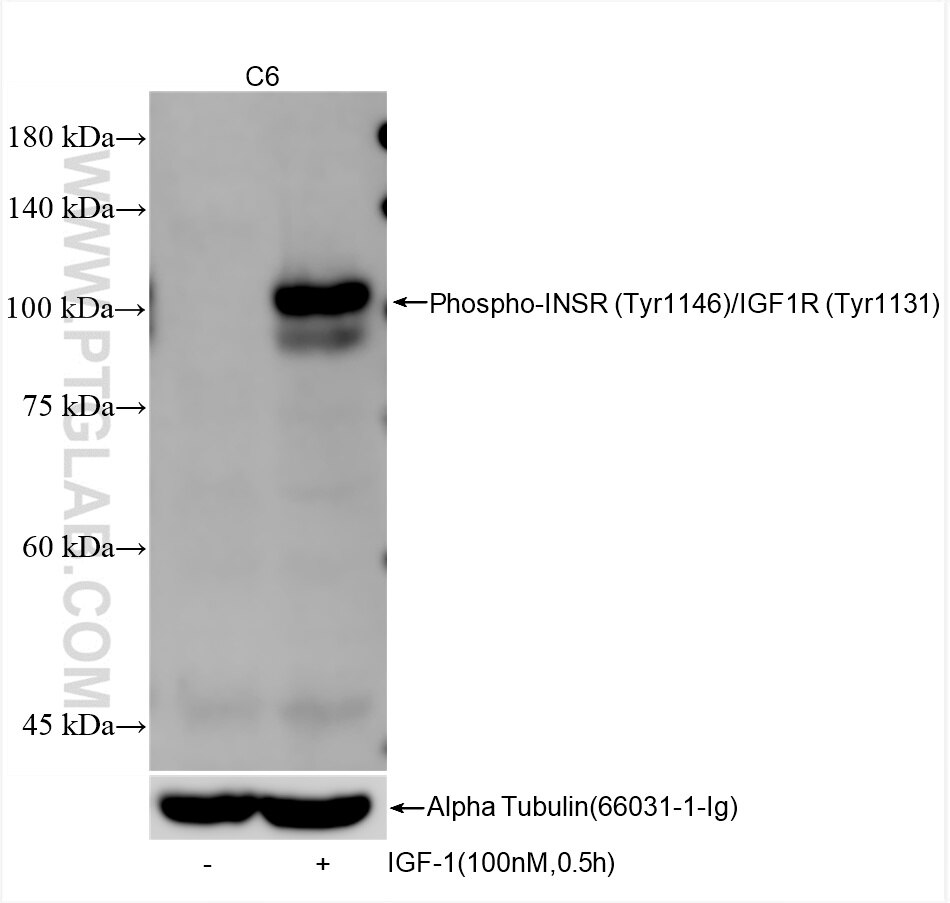
82830-15-PBS targets Phospho-INSR (Tyr1146)/IGF1R (Tyr1131) in WB, Indirect ELISA applications and shows reactivity with human, mouse, rat samples.
| Tested Reactivity | human, mouse, rat |
| Host / Isotype | Rabbit / IgG |
| Class | Recombinant |
| Type | Antibody |
| Immunogen |
Peptide Predict reactive species |
| Full Name | INSR |
| Observed Molecular Weight | 95 kDa |
| GenBank Accession Number | BC117172 |
| Gene Symbol | INSR |
| Gene ID (NCBI) | 3643 |
| Conjugate | Unconjugated |
| Form | Liquid |
| Purification Method | Protein A purification |
| UNIPROT ID | P06213 |
| Storage Buffer | PBS only, pH 7.3. |
| Storage Conditions | Store at -80°C. |
Background Information
Insulin binding to the insulin receptor (INSR) triggers sequential conformational changes and autophosphorylation of the receptor, followed by activation of a kinase signaling cascade that plays essential roles in a wide variety of biological processes. INSR belongs to a class of receptor tyrosine kinases (RTKs) that comprises 58 receptors in humans. The INSR shares a high structural homology with the IGF1R (84% similarity in the tyrosine kinase domain, 45-65% in the ligand-binding domain, and more than 50% in the overall amino acid sequence). In addition, ligand-dependent activation of the INSR and IGF1R activates almost identical downstream signaling cascades. Insulin binds to INSR in peripheral tissues, initiating receptor activation followed by intracellular signaling cascades. The first step in INSR activation is the autophosphorylation of intracellular tyrosine residues in the JM domain, kinase activation loop, and CT domain. Phosphorylation of three tyrosine residues (Tyr1146, Tyr1150, and Tyr1151, based on INSR isoform A numbering) located in the kinase activation loop plays a crucial role in kinase activity regulation. Insulin binding also induces INSR kinase-mediated phosphorylation of four tyrosine residues located in the JM (Tyr953 and Tyr960) and CT domain (Tyr1316 and Tyr1322). (PMID: 37779149,PMID:24434591)