Actin Chromobody for live-cell super-resolution imaging
The Actin Chromobody enables non-invasive labeling of actin microfilaments
The ChromoTek Actin Chromobody is a live-cell probe for visualization of the actin cytoskeleton and monitoring its dynamics. The Actin Chromobody enables non-invasive labeling of actin microfilaments not only in mammalian cells, but also in cells and tissues of evolutionary distant species, such as Zebrafish (Panza et al. 2015) or plants (Rocchetti, Hawes, and Kriechbaumer 2014). The Z-stack shows confocal images of optical sections of Actin Chromobody in a Hela cell.
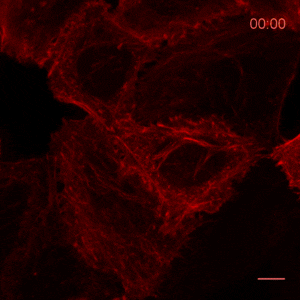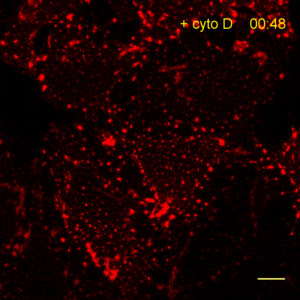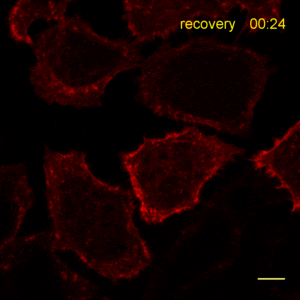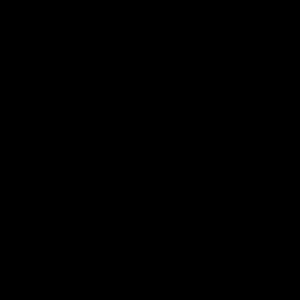
Recently, the Actin Chromobody was imaged in living cells using super-resolution STED microscopy by Stefan Hell’s group at the MPI bpc in Goettingen. Their study “Coordinate-targeted fluorescence nanoscopy with multiple off states” was published in Nature Photonics (Danzl et al. 2016). This article describes the implementation of a novel multiple off state transitions concept for enhancing resolution and reducing bleaching in live-cell STED imaging. The novel so-called “protected STED” mode utilizes reversibly photoswitchable fluorescent proteins, such as rsEGFP variants, as genetic tags. The authors applied the newly developed method to super-resolve a cortical actin network labeled with the ChromoTek Actin Chromobody in living cultured fibroblasts (see Fig. 5 and Suppl. Fig. 20, in (Danzl et al. 2016)). Besides, they imaged a 3D arrangement of microfilaments labeled with the Actin Chromobody in living hippocampal neurons of organotypic hippocampal brain slices and super-resolved dendritic spines emanating from a dendritic shaft (see Fig. 6., Suppl. Fig. 21, and Suppl. Movie 5, in (Danzl et al. 2016)).
These imaging improvements can tremendously enhance the applicability of STED microscopy for live-cell imaging, which so far has been hindered by severe phototoxicity and photobleaching. The authors envision that the “protected STED” method will pave the way for many future live-cell microscopy studies with diffraction-unlimited 3D resolution.
References:
Danzl, Johann G., Sven C. Sidenstein, Carola Gregor, Nicolai T. Urban, Peter Ilgen, Stefan Jakobs, and Stefan W. Hell. 2016. 'Coordinate-targeted fluorescence nanoscopy with multiple off states', Nat Photon, 10: 122-28.
Panza, P., J. Maier, C. Schmees, U. Rothbauer, and C. Sollner. 2015. 'Live imaging of endogenous protein dynamics in zebrafish using chromobodies', Development, 142: 1879-84.
Rocchetti, A., C. Hawes, and V. Kriechbaumer. 2014. 'Fluorescent labelling of the actin cytoskeleton in plants using a cameloid antibody', Plant Methods, 10: 12.
Related Content
Nanobody-based reagents by ChromoTek
Intracellular Nanobodies for live-cell analysis in real-time
Chromobodies for live cell imaging
Visualize Histones in live cells: Histone-Chromobody
Visualize the onset of metastasis with Chromobodies
Actin Chromobody: Visualization of actin dynamics – NOT alteration
Novel cell cycle modulators identified using the Cell Cycle Chromobody Technology
