Ferritinophagy: Understanding Its Roles in Iron Homeostasis and Disease
Written by Elizabeth Byers, PhD Candidate at The Pennsylvania State University
What is Ferritinophagy
Ferritinophagy is a selective form of autophagy involved in regulating intracellular iron homeostasis through the degradation of ferritin, an iron storage protein1,2. To understand what the process is and how it works, we must first understand a bit about how iron is stored and used in the body.
Iron is necessary for many biological processes. A lack of iron can cause anemia, resulting in a deficiency of healthy red blood cells or hemoglobin necessary to carry oxygen to the body’s tissues. This can cause health problems, especially in children, by affecting their immune systems3. However, too much iron can also be dangerous through the generation of reactive oxygen species (ROS)4 which can cause cell damage and trigger inflammation.
To prevent this, the body can store iron in proteins to reduce its reactivity, while allowing it to be used when needed – for example, in metalloprotein synthesis. Overall, a tight balance must be struck to maintain proper iron homeostasis.
Autophagy is a key cellular process that maintains homeostasis by recycling damaged, misfolded, or unneeded components. Through this intracellular degradation system, cytoplasmic materials are delivered to the lysosome for degradation. More than just a waste disposal mechanism, autophagy serves as a dynamic recycling system that produces new building blocks and energy for cellular renovation and homeostasis.
Autophagy was first implicated in iron homeostasis in 2011, when Asano et. al discovered ferritin was targeted for degradation in the lysosome via a mechanism that involved autophagy in cells lacking sufficient iron5. Since then, the role of autophagy in ferritin degradation has been confirmed through autophagy deletion models where ferritin clusters build up in cells6. Researchers have also identified that the protein nuclear receptor co-activator 4 (NCOA4) helps guide ferritin to the lysosome for breakdown7,8. This specific type of autophagy has been named ferritinophagy. If it doesn’t work properly and excessive intracellular iron accumulates, it can lead to ferroptosis, a type of controlled cell death caused by excess iron.
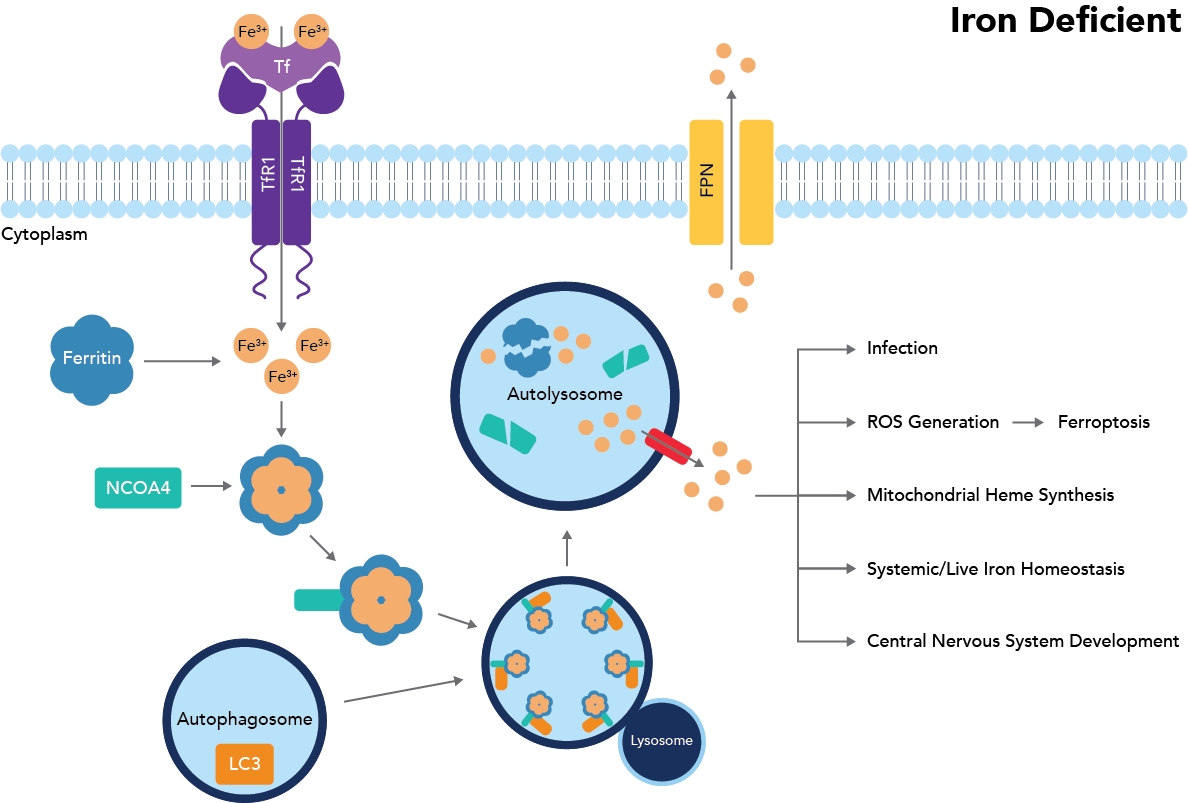
Figure 1: Ferritinophagy pathway
Pathway and Important Signaling Proteins
Ferritinophagy is triggered when intracellular iron concentrations drop below a certain threshold. Specifically, breaking down ferritin is a potent way to raise free iron concentrations, as this protein, made up of 24 subunits of ferritin light (FTL) and heavy (FTH1) chains, can bind and sequester between 2,000-2,500 iron atoms per ferritin protein9. The current understanding of the ferritinophagy pathway is diagramed in Fig. 1. Key proteins in this signaling cascade are those involved in iron transport, import, export, and storage.
Transferrin (Tf) and the transferrin receptor (TfR1/CD71) are critical to bind and transport iron into the cell, where it can then be passed to ferritin for storage. Ferritin specifically houses iron as Fe(III) to prevent further iron oxidation and the formation of ROS. If iron is needed, it may either exit the protein through pore structures (although this is much less frequent) or be released through ferritin breakdown. To break down ferritin, NCOA4 binds FTH1 on ferritin and transports it to the autophagosome. Once in the autophagosome, the NCOA4-FTH1 complex binds LC3, which is moved to the autolysosome. In the autolysosome, ferritin is finally degraded to release iron1. If, instead, there is too much free intracellular iron, this iron will bind to ferritin and prevent NCOA4 binding of FTH1, as iron and NCOA4 are suspected to compete for the same binding site. Instead, NCOA4 will bind the E3 ubiquitin ligase HERC2 and be transported to the proteosome for degradation, thereby preventing ferritinophagy from occurring and leading to increased iron storage (Fig. 2)2.
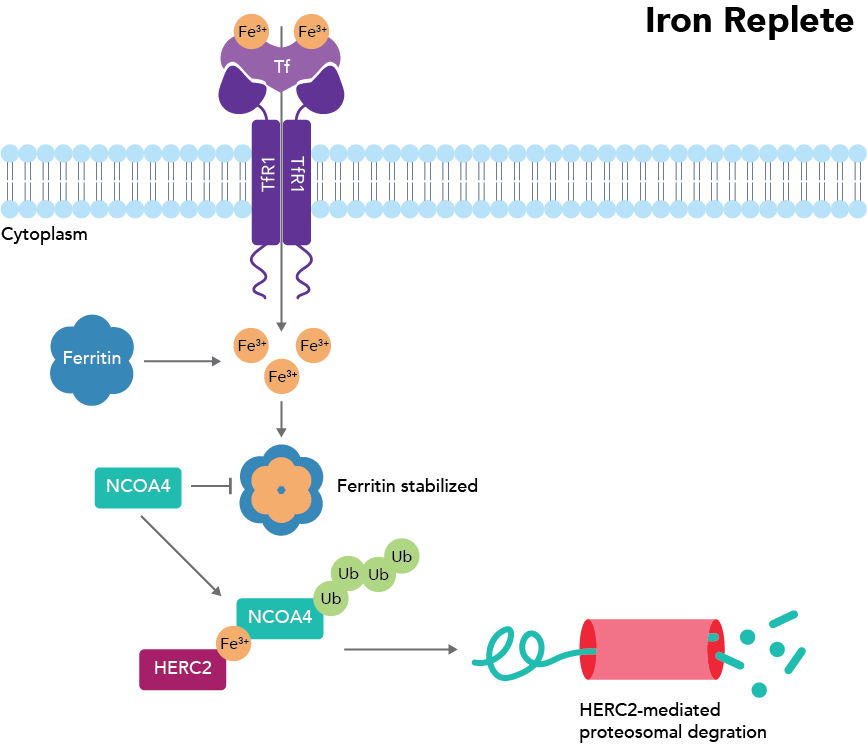
Figure 2: Iron-deficient situation where ferritinophagy does not occur. Adapted from Santana-Codina et al., 2018.
The iron export transporter ferroportin (FPN) further influences this balance by contributing to iron removal through export to the plasma, thereby reducing the pool of free iron inside the cell10. Expression levels and regulation of FPN vary by cell type, depending on their function and iron handling needs11. By controlling how much free iron is present, FPN indirectly stabilizes NCOA4, enabling ferritinophagy to proceed. Thus, FPN serves as an additional layer of control in maintaining iron homeostasis through its impact on NCOA4 activity and ferritin turnover.
Research Areas and Associated Pathologies
Despite growing insights, many aspects of ferritinophagy and ferritin recycling are still not fully understood. Although it appears that classical autophagy processes are responsible for transporting NCOA4-FTH1 to the lysosome, other pathways may help release iron from ferritin. Potential mechanisms may include the ubiquitin-proteosome axis, ESCRT complex-mediated endosomal sorting, or ESCRT-III endosomal sorting2. This research has the potential to impact many other branches of inquiry, as ferritinophagy has been found to play an important role in fundamental cell processes such as cell differentiation, erythropoiesis, and the immune response. Figure 3 illustrates how ferritinophagy fits into the grander scheme of iron flux in the body12.
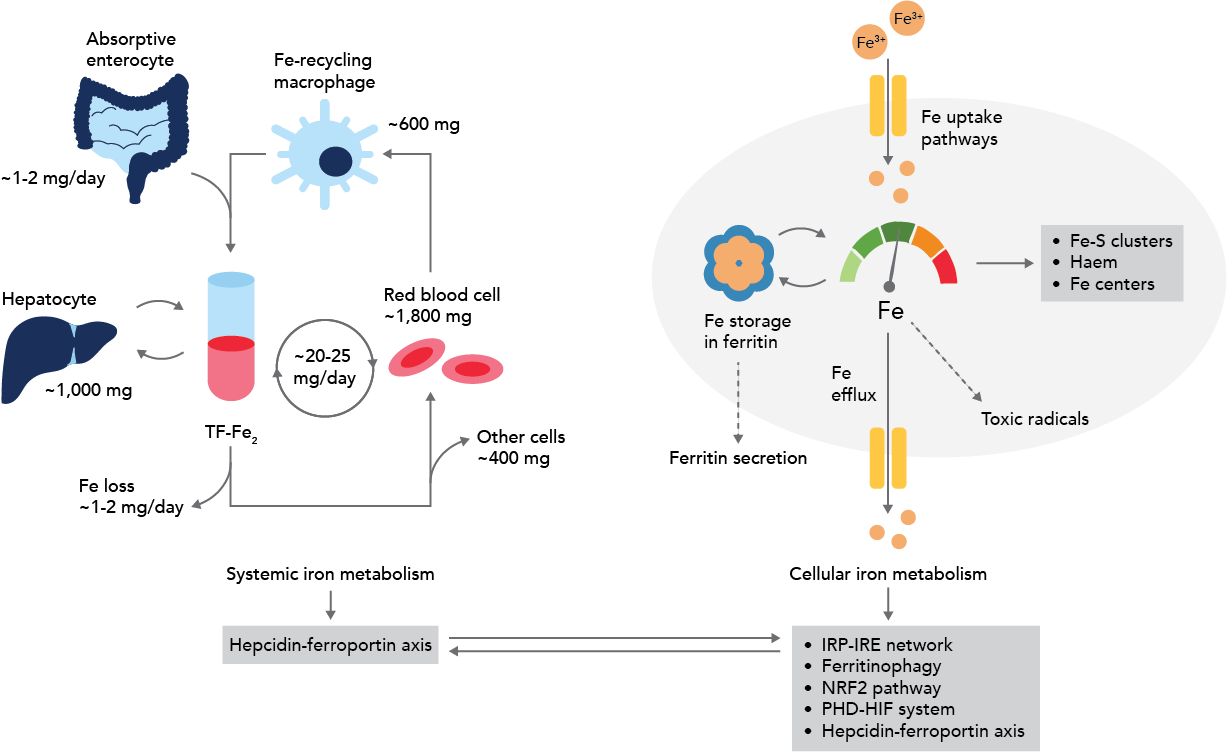
Figure 3: Iron flux and ferritinophagy. Adapted from Galy et al., 2023.
If ferritinophagy proceeds unchecked, it can raise intracellular iron levels and elevate the likelihood of ROS production through the Fenton reaction, in which iron reacts with hydrogen peroxide to generate highly damaging hydroxyl radicals. Polyunsaturated fatty acids, the main component of membrane lipids, are particularly at risk for ROS damage, and peroxidized lipids in the plasma membrane can lead to rupture and cell death via ferroptosis13.
Dysregulated ferritinophagy plays a role in a wide variety of pathologies. These range from cancer, neurodegenerative diseases such as Parkinson’s and Alzheimer’s, and iron overload disorders like hemochromatosis and iron overload cardiomyopathy14.
Cancer. Two key proteins in the ferritinophagy pathway, FPN and TfR1, can become dysregulated in cancer. In ovarian cancer specifically, FPN levels decrease while TfR1 levels increase, leading to a buildup of iron within the cell. Elevated TfR1 expression has also been observed in lung and cervical cancer, as well as hepatocellular carcinoma1, making it a potential marker for these cancers.
What is perhaps even more compelling than ferritinophagy’s role in cancer progression is its potential as a therapeutic target. Since ferritinophagy acts upstream of ferroptosis, it may be harnessed to selectively kill cancer cells. Several studies have investigated using compounds like vitamin C15, caryophyllene oxide16, dihydroartemisinin (an anti-malarial drug)17, and esculetin (a coumarin compound found in many plants, spices, and foods such as cinnamon, green tea, carrots, and beer)18 as inducers of ferritinophagy to trigger cancer cell death through iron overload.
Neurodegenerative Disorders. In Parkinson’s Disease, substantial increases in iron concentrations can be found in the substantia nigra and globus pallidus, two regions of the brain with important roles in the disease’s progression. In Alzheimer’s Disease, increased iron concentration can also be detrimental via upregulating amyloid precursor protein (APP) expression. APP breakdown makes amyloid-β, therefore, elevated APP production raises the risk of amyloid-β plaque formation, a hallmark of Alzheimer’s19.
Hemochromatosis. In contrast to cancer and neurodegenerative disorders, which can develop over time due to multiple factors, hemochromatosis is a hereditary genetic disorder most often caused by a homozygous mutation in hepcidin, a hormone that regulates the activity of FPN. Normally, hepcidin binds FPN and prevents iron release into the plasma, but in the disease state, reduced amounts of hepcidin lead to iron overload in the blood, which is then passed into hepatocytes, pancreatic cells, and cardiomyocytes20. An overabundance of iron in cardiomyocytes, in particular, can precipitate cardiomyopathy and heart failure by causing mitochondrial dysfunction, ROS formation, and triggering ferroptosis14.
Pathogens. Additionally, viruses and bacteria can also utilize ferritinophagy in their life cycles. Human parainfluenza virus type 2 can hijack the ferritinophagy pathway for viral replication, and Ehrlichia chaffeensis, a bacterium that causes a potentially fatal tick-borne disease, uropathogenic Escherichia coli, and Brucella similarly use the pathway to increase iron availability for bacterial growth12,14,21.
With the discovery of ferritinophagy and particularly the associated cell death mechanism of ferroptosis, research into iron’s role in regulating cell survival has become more popular. In some fields, researchers are interested in how the processes can be utilized as a new treatment target and kill switch, but in others, restricting ferritinophagy, iron release, and the resulting iron overload is the focus. Much remains to be learned!
References
- Wang, J. et al. Ferritinophagy: research advance and clinical significance in cancers. Cell Death Discovery 9, 1–10 (2023).
- Santana-Codina, N. & Mancias, J. D. The role of NCOA4-mediated ferritinophagy in health and disease. Pharmaceuticals 11, 114 (2018).
- Peroni, D. G., Hufnagl, K., Comberiati, P. & Roth-Walter, F. Lack of iron, zinc, and vitamins as a contributor to the etiology of atopic diseases. Frontiers in Nutrition vol. 9 Preprint at https://doi.org/10.3389/fnut.2022.1032481 (2023).
- Winterbourn, C. C. The Biological Chemistry of Hydrogen Peroxide. Methods in Enzymology 528, 3–25 (2013).
- Asano, T. et al. Distinct mechanisms of ferritin delivery to lysosomes in iron-depleted and iron-replete cells. Molecular and Cellular Biology 31, 2040–2052 (2011).
- Kishi-Itakura, C., Koyama-Honda, I., Itakura, E. & Mizushima, N. Ultrastructural analysis of autophagosome organization using mammalian autophagy-deficient cells. Journal of Cell Science 127, 4984–4984 (2014).
- Dowdle, W. E. et al. Selective VPS34 inhibitor blocks autophagy and uncovers a role for NCOA4 in ferritin degradation and iron homeostasis in vivo. Nature Cell Biology 16, 1069–1079 (2014).
- Mancias, J. D., Wang, X., Gygi, S. P., Harper, J. W. & Kimmelman, A. C. Quantitative proteomics identifies NCOA4 as the cargo receptor mediating ferritinophagy. Nature 509, 105–109 (2014).
- Kaur, J. & Debnath, J. Autophagy at the crossroads of catabolism and anabolism. Nature Reviews Molecular Cell Biology 16, 461–472 (2015).
- De Domenico, I. et al. Ferroportin-mediated mobilization of ferritin iron precedes ferritin degradation by the proteasome. The EMBO Journal 25, 5396 (2006).
- Drakesmith, H., Nemeth, E. & Ganz, T. Ironing out Ferroportin. Cell Metabolism 22, 777–787 (2015).
- Galy, B., Conrad, M. & Muckenthaler, M. Mechanisms controlling cellular and systemic iron homeostasis. Nature Reviews Molecular Cell Biology 25, 133–155 (2023).
- Belik, J. Susceptibility of the Immature Lung to Oxidative and Mechanical Injury. The Newborn Lung: Neonatology Questions and Controversies 101–118 (2008) doi.org/10.1016/B978-141603166-6.10005-1.
- Liu, M. Z. et al. The critical role of ferritinophagy in human disease. Frontiers in Pharmacology 13, 933732 (2022).
- Wang, X. et al. Vitamin C induces ferroptosis in anaplastic thyroid cancer cells by ferritinophagy activation. Biochemical and Biophysical Research Communications 551, 46-53 (2021).
- Xiu, Z. et al. Caryophyllene Oxide Induces Ferritinophagy by Regulating the NCOA4/FTH1/LC3 Pathway in Hepatocellular Carcinoma. Frontiers in Pharmacology 13, 930958 (2022).
- Shi, H. et al. Susceptibility of cervical cancer to dihydroartemisinin-induced ferritinophagy-dependent ferroptosis. Frontiers in Molecular Biosciences 10, 1156062 (2023).
- Xiu, Z. et al. Inhibitory Effects of Esculetin on Liver Cancer Through Triggering NCOA4 Pathway-Mediation Ferritinophagy in vivo and in vitro. Journal of Hepatocell Carcinoma 10, 611-629 (2023).
- Li, S. et al. Mechanisms of Ferritinophagy and Ferroptosis in Diseases. Molecular Neurobiology 2023 61, 1605–1626 (2023).
- Brissot, P. et al. Haemochromatosis. Nature Reviews Disease Primers 4, 18016 (2018).
- Hop, H. T., Huy, T. X. N., Lee, H. J. & Kim, S. Intracellular growth of Brucella is mediated by Dps‐dependent activation of ferritinophagy. EMBO Reports 24, e55376 (2023).
Related Content
Autophagy and Cell Death Portfolio
The Role of Autophagy in Cancer
The role of ferroptosis in human diseases
What is the difference between pyroptosis and apoptosis?
Support
Newsletter Signup
Stay up-to-date with our latest news and events. New to Proteintech? Get 10% off your first order when you sign up.

