Important facts to know when using Nano-Secondary reagents
What I should know when I'm working with Secondary antibodies or Nano-Secondary reagents
What is a Nano-Secondary reagent?
ChromoTek’s Nano-Secondary reagents are novel secondary antibodies for higher resolution, cleaner images, and faster immunostaining. Nano-Secondary reagents consist of alpaca Nanobodies/ VHHs that bind to primary antibodies with high affinity in a species and subclass-specific manner. Nano-Secondary reagents are conjugated to Alexa Fluor® dyes. Currently, Nano-Secondary reagents that bind to anti-rabbit or subclass-specific anti-mouse IgGs are available.
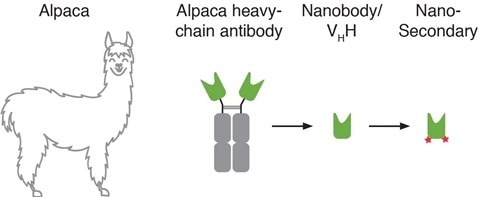
Figure 1: Nano-Secondary reagents are alpaca Nanobodies conjugated to fluorescent dyes that specifically bind to primary antibodies.
What is one-step immunostaining?
One-step immunostaining is the simultaneous incubation of primary antibody and Nano-Secondary reagent. This method reduces incubation time, hands-on time and the number of washing steps. The simultaneous incubation of primary antibody and Nano-Secondary reagent also supports multiplexing, live-cell immunostaining, and improves cell viability for flow cytometric analysis.
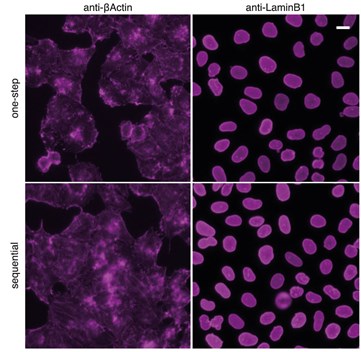
Figure 2: Comparison with classical sequential staining of primary antibody and Nano-Secondary reagent: HeLa cells were immunostained with rabbit anti-βActin and anti-LaminB1 and secondary anti-rabbit IgG VHH Alexa Fluor647. Scale bar, 20 µm. Epifluorescence images were acquired with CellInsight CX7 HCS microscope, 20X objective.
How does one-step immunostaining work?
Because the Secondary reagents are monovalent and bind with high specificity and affinity to their target IgGs, they can be simultaneously incubated with the primary antibody. This results in a one-step immunostaining. In practical terms, the Nano-Secondary reagent is added to the primary antibody and the primary antibody’s protocol is used for immunostaining.

Figure 3: Experimental procedure of one-step immunostaining
Is there a protocol for one-step immunostaining for immunofluorescence?
Yes, please find the protocol here: One-step immunostaining for immunofluorescence
For what applications can I use Nano-Secondary reagents?
One-step or parallel incubation of primary antibody and corresponding Nano-Secondary reagent is used for immunofluorescence, super-resolution microscopy, Western blotting, and flow cytometry.
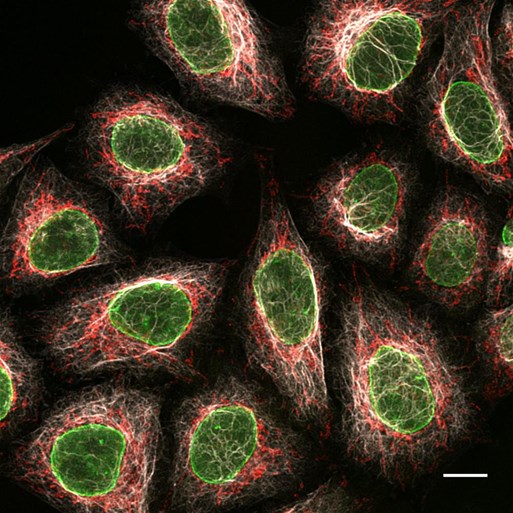
Figure 4: Multiplexing immunofluorescence a using triple mouse secondary antibody staining: 3 subclass specific anti-mouse Nano-Secondary reagents: Immunofluorescence/ confocal microscopy of HeLa cells with alpaca anti-rabbit Nano-Secondary reagents: From left to right Mouse IgG2b anti-Lamin + alpaca anti-mouse IgG2b VHH Alexa Fluor 488; Mouse IgG3 anti-MOT + alpaca anti-mouse IgG3 VHH Alexa Fluor 568; Mouse IgG1 anti-Vimentin + alpaca anti-mouse IgG1 VHH Alexa Fluor 647. Scale bar, 20 μm. Confocal images were acquired with a Leica TCS SP8 microscope, 100x oil objective, and deconvolved with Huygens Professional (SVI). Images were recorded at the Core Facility Bioimaging at the Biomedical Center, LMU Munich.
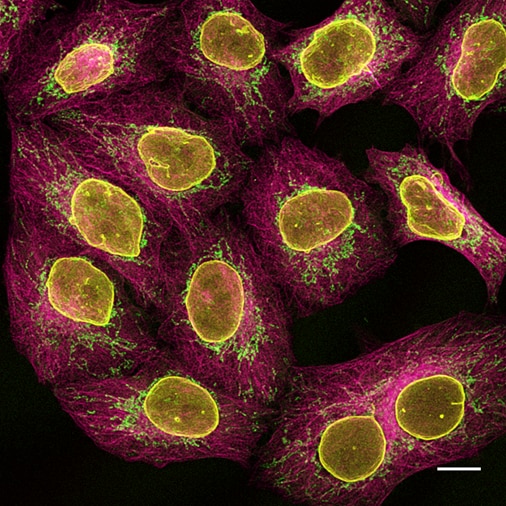
Figure 5: Multiplexing of HeLa cells with alpaca anti-rabbit and anti-mouse Nano-Secondary reagents. Yellow: rabbit anti-Lamin + alpaca anti-rabbit IgG VHH Alexa Fluor 568. Green: mouse IgG1 anti-COX4 + alpaca anti-mouse IgG1 VHH Alexa Fluor 488. Magenta: mouse IgG2b anti-Tubulin + alpaca anti-mouse IgG2b VHH Alexa Fluor 647. Scale bar, 10 μm. Confocal images were acquired with a Leica TCS SP8 microscope, 100x oil objective, and deconvolved with Huygens Professional (SVI). Images were recorded at the Core Facility Bioimaging at the Biomedical Center, LMU Munich.
Does multiplex fluorescent Western blotting work with Nano-Secondary reagents?
Yes, multiple Nano-Secondary reagents can be applied in parallel for multiplex fluorescent Western blotting. This allows multiple targets to be analyzed simultaneously on the same blot at the same time. This way, it is not necessary to strip and re-probe the Western blot membrane. In practical terms, the Nano-Secondary is mixed with the primary antibody and the primary antibody’s protocol is used for Western blotting.
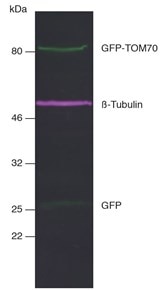
Figure 6: Multiplex fluorescent Western blot of GFP-TOM70, ß-Tubulin, and GFP in HEK293T cell lysate: Membrane was simultaneously incubated with primary antibodies and Nano-Secondary reagents. Green: rabbit anti-GFP (ChromoTek PABG1) + alpaca anti-rabbit IgG VHH Alexa Fluor 488, magenta: mouse anti-ß-Tubulin + alpaca anti-mouse IgG2b VHH Alexa Fluor 647.
What is the size of Nano-Secondary reagents?
Nano-Secondary reagents are about 15 kDa in size and thus 10 times smaller than conventional secondary antibodies or IgGs, which are ≥160 kDa. That small size considerably improves tissue penetration and decreases the distance between epitope and label, which increases imaging resolution. Hence, Nano-Secondary reagents are perfect probes for super-resolution microscopy, e.g. STED, STORM, etc. Also, for tissue, organ, and whole animal imaging the Nano-Secondary reagents are beneficial due to their small size.
Do I need to know what subclass my primary antibody is?
Yes, because Nano-Secondary reagents are subclass-specific, i.e. they bind to mouse IgG1, IgG2b, and IgG3. Rabbit does not have IgG subclasses – thus, the anti-rabbit Nano-Secondary reagents do bind to all rabbit primary antibodies.
What is the specificity or target of Nano-Secondary reagents?
Nano-Secondary reagents are subclass-specific and do not cross-react with IgGs from other commonly used species. During product development, we exclude Nanobodies that cross-react to other commonly used species’ IgGs. Tested species are reported in the product documentation; see here for more details. We select only Nanobodies with the desired specificity. Therefore, our Nano-Secondary reagents have a very low background and do not require any kind of pre-adsorption. Currently, Nano-Secondary reagents against rabbit IgG plus mouse IgG1, IgG2b, and IgG3 are available.
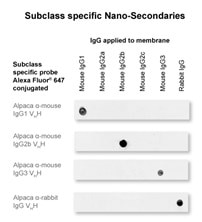
Figure 7: Nano-Secondary reagents are subclass-specific and do not cross-react with IgGs from other commonly used species: This high specificity in combination with low background enables multiplexing. Note competitor’s cross-reactivity to mouse IgGs despite of pre-adsorption against mouse serum.
How do Nano-Secondary reagents bind to primary antibodies?
Nano-Secondary reagents enable very precise stainings as they bind in a site-specific manner to primary IgG antibodies: they bind either to the Fc or Fab region of the primary antibody, as specified in the product documentation. In general, they are fully characterized and validated.
Figure 8: Site-specific staining of primary antibodies: Anti-rabbit Nano-Secondary consists of 2 monoclonal Nanobodies that specifically bind to the Fc-region and Fab-region of the rabbit primary antibody. Both Nanobodies are stoichiometrically labelled with 2 Alexa Fluor® dyes each. In total 4 Nanobodies bind per rabbit primary antibody. For anti-mouse Nano-Secondary reagents, site-specific binding of Nanobodies and their stoichiometric labelling is determined, too; these vary by subclass primary antibody and are reported in the product documentation. Please click here for more details.
What conjugations are available for Nano-Secondary reagents?
Nano-Secondary reagents are stoichiometrically labeled with Alexa Fluor® dyes. Dependent on the Nanobody of the actual Nano-Secondary, two or three Alexa Fluor fluorophores are conjugated to the Nanobody as specified in the product documentation.
How are Nano-Secondary reagents manufactured?
Nano-Secondary reagents are totally animal free manufactured, though the VHHs are generated from immunization of alpacas. Their recombinant production in combination with high QC standards ensures reliable and stable alpaca single domain antibody products virtually without lot-to-lot variations. Furthermore, they are available at unlimited supply.
How are Nano-Secondary reagents characterized and validated?
ChromoTek utilizes biochemical strategies and comparison with independent secondary antibodies for the validation of our Nano-Secondary reagents.
- In the biochemical approach, the Nano-Secondary reagents are tested in immunofluorescence and Western blotting applications both using cells with and without their cognate IgG primary antibody.
- In addition, our Nano-Secondary reagents are benchmarked with established conventional secondary antibodies.
Our Nano-Secondary reagents are sequenced. In addition, we thoroughly characterize and validate our monoclonal Nano-Secondary reagents: we determine cross-reactivity, sequence, affinity, melting point, and degree of labeling (DOL). Please click here for more details.
Figure 9: Discussion on Primary Antibodies Extends to Secondary Antibodies: sources: journals and CiteAb blog.
What is a Nanobody or VHH?
Is there really still need for an explanation? Ok, here you go:
In addition to conventional IgG antibodies, camelids like alpacas, lamas and dromedary also possess heavy chain only IgGs. These antibodies lack the CH1 domain of the heavy chain and are devoid of light chains. Their antigen binding domain is built up solely by their heavy chain and is called VHH or Nanobody.
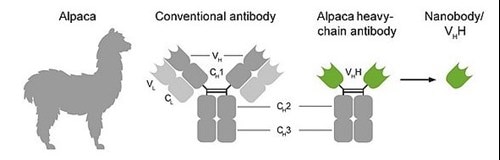
Figure 10: Origin of Nanobodies and comparison with conventional antibodies. Please click here for more details.
Related Content
Why are recombinant Nanobodies/ VHHs beneficial?
Advantages of recombinant Nano-Secondary reagents
Important facts to know when using Nano-Secondary reagents
The importance of blocking when using Nano-Secondary reagents for IF
Want to upgrade your immunofluorescence workflow? Go Direct!

Support
Newsletter Signup
Stay up-to-date with our latest news and events. New to Proteintech? Get 10% off your first order when you sign up.
