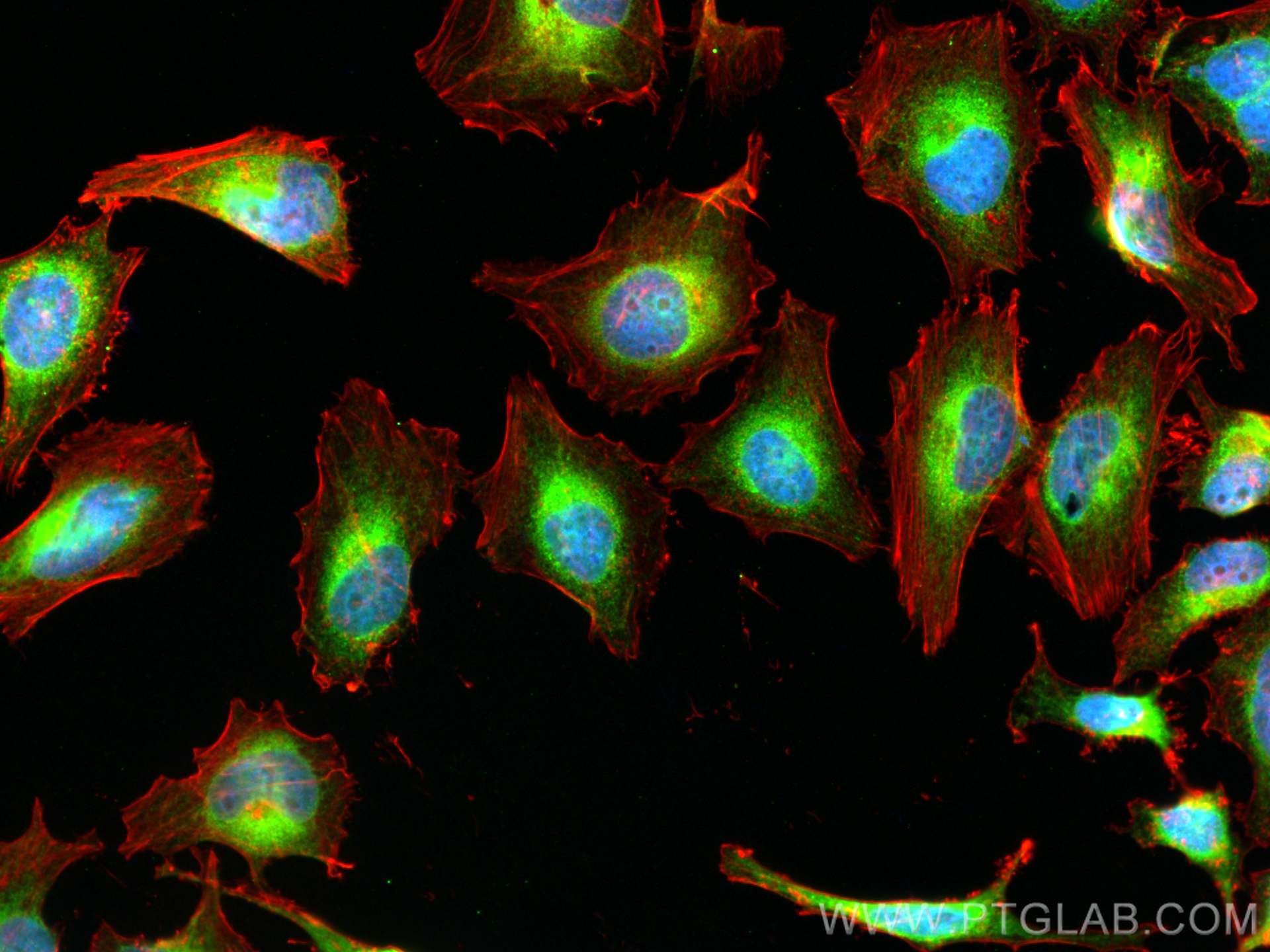
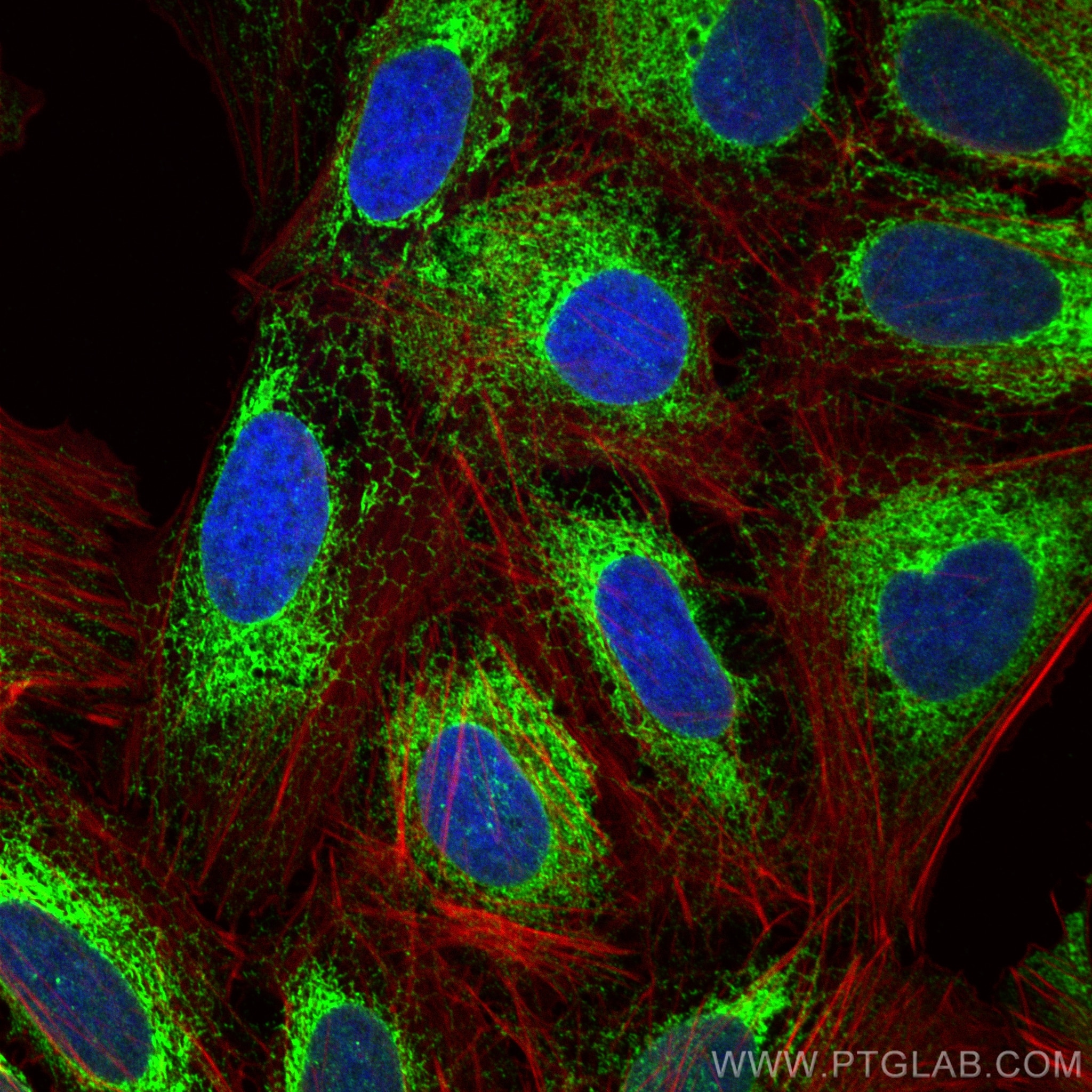
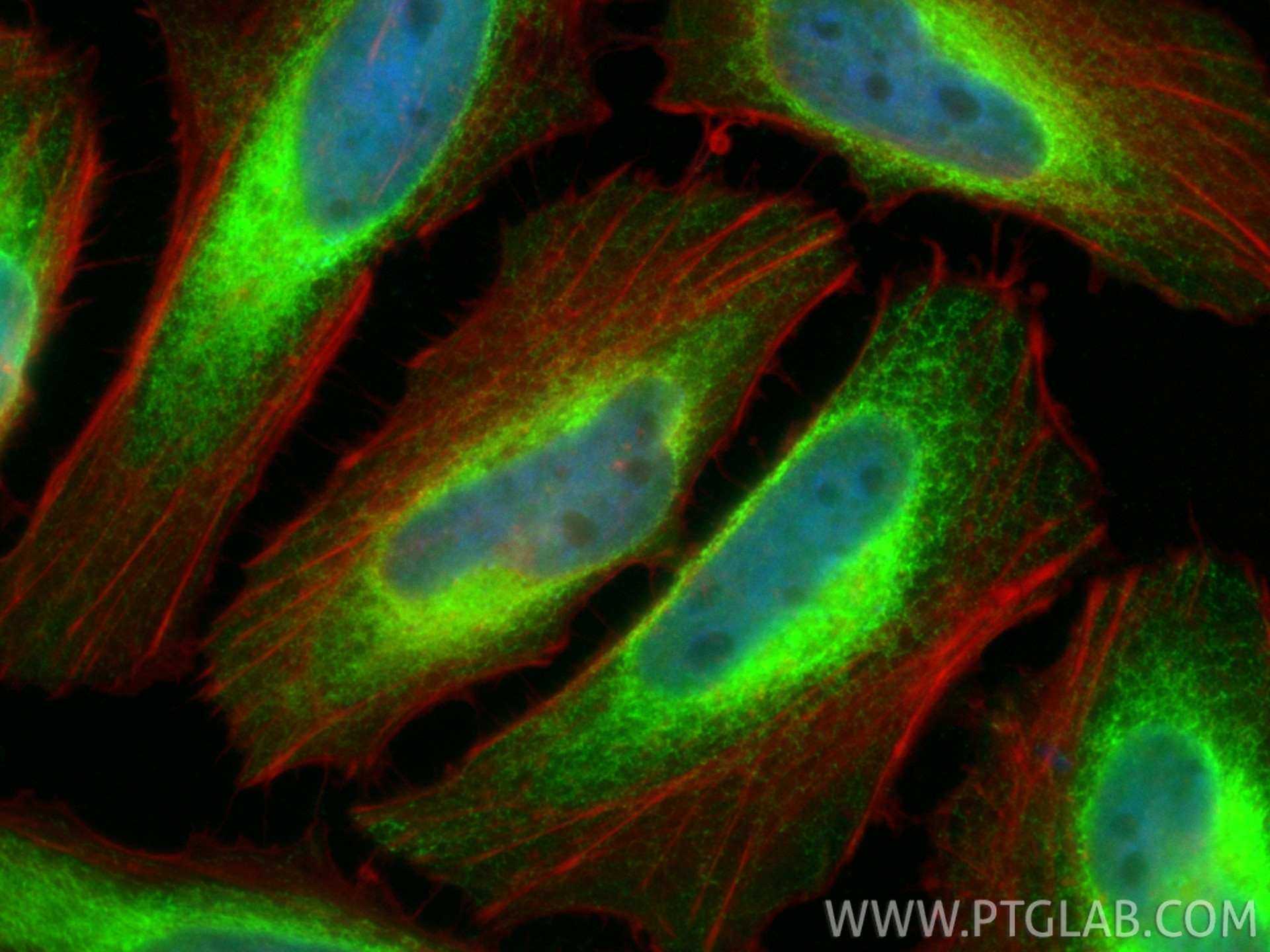
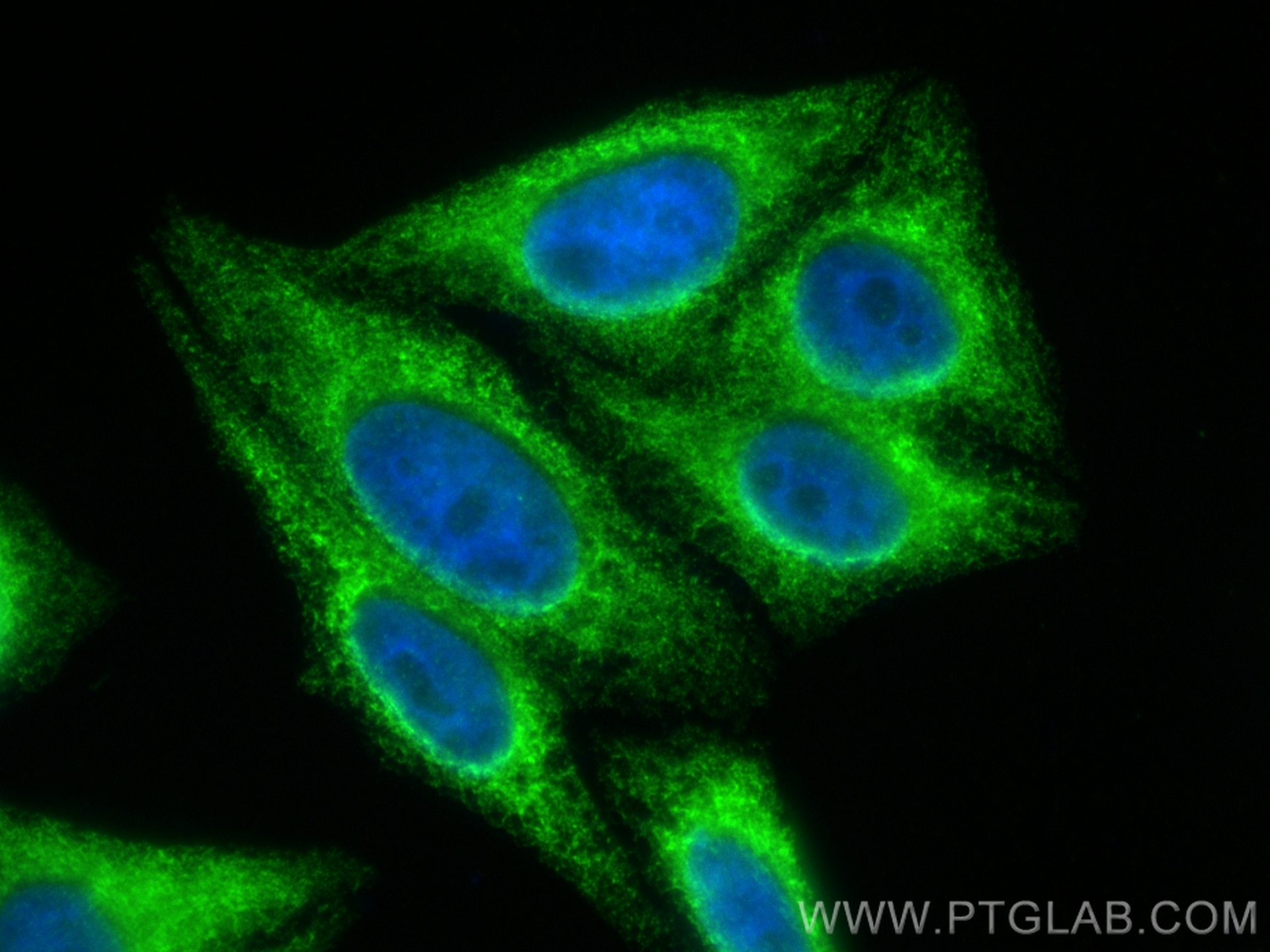
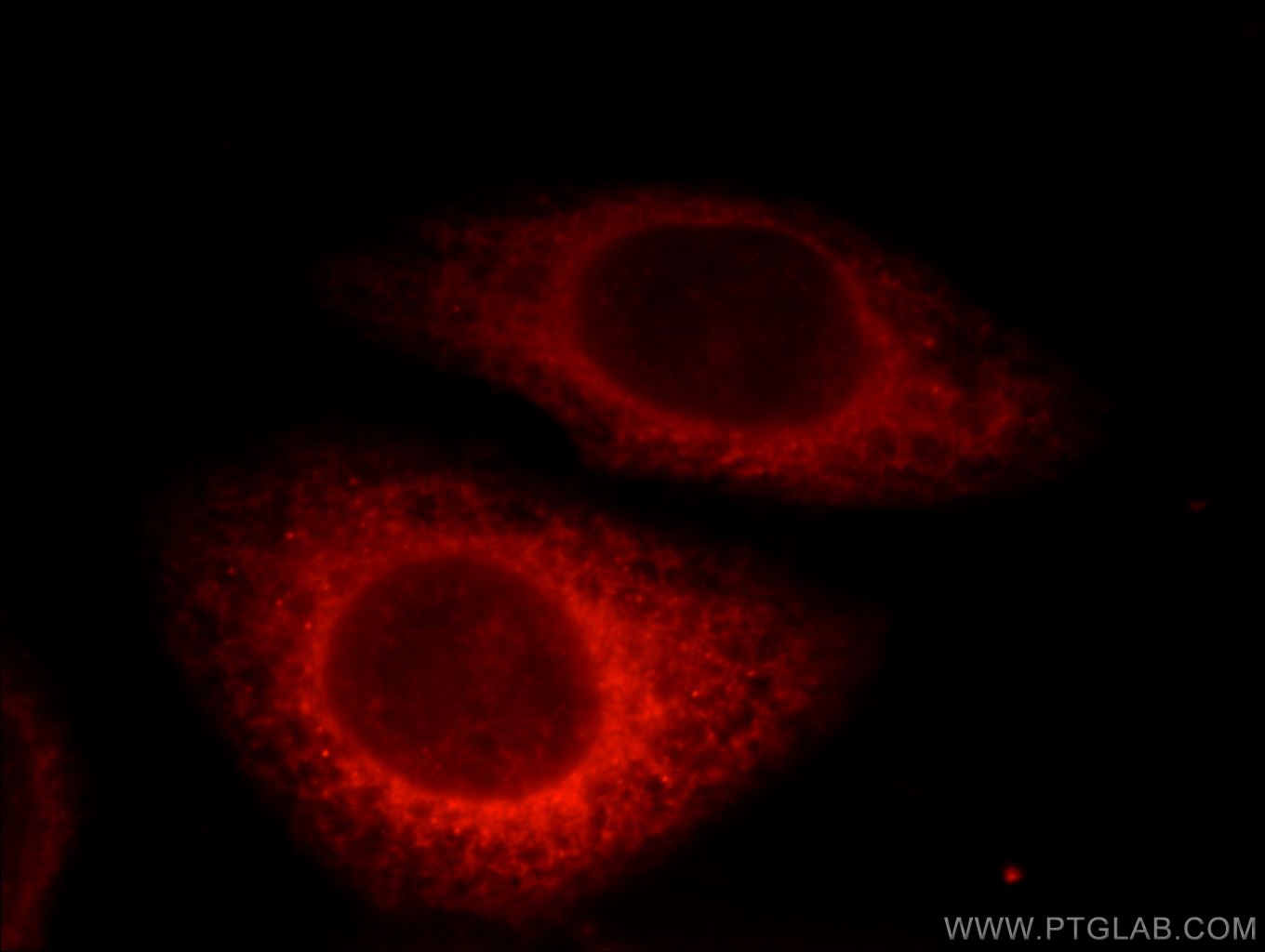
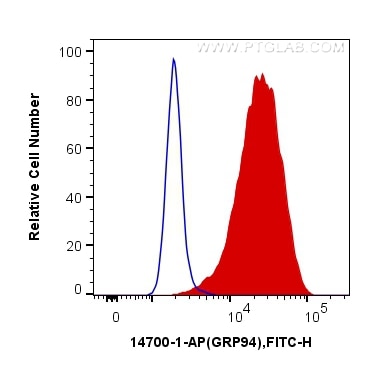
Tested Applications
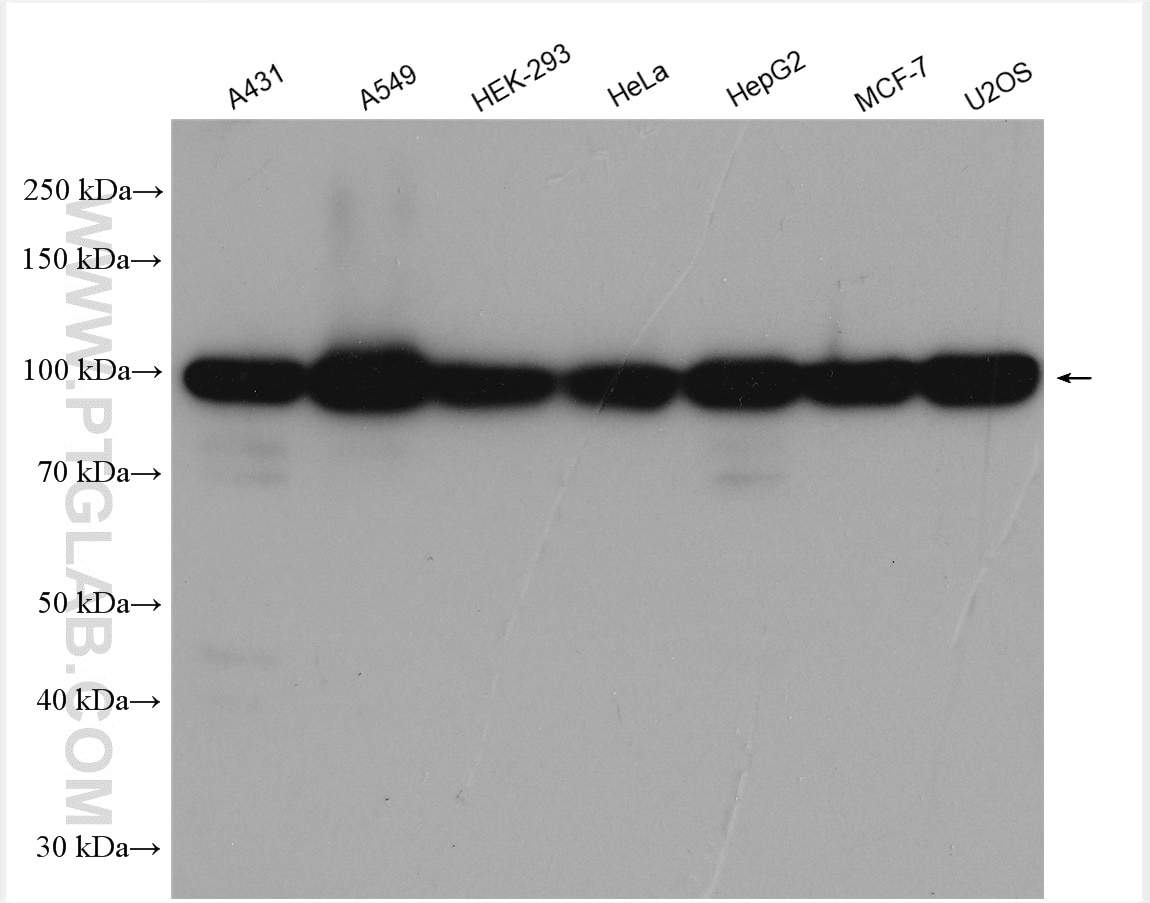
| Positive WB detected in | A431 cells, HEK-293 cells, A549 cells, HeLa cells, HepG2 cells, MCF-7 cells, U2OS cells |
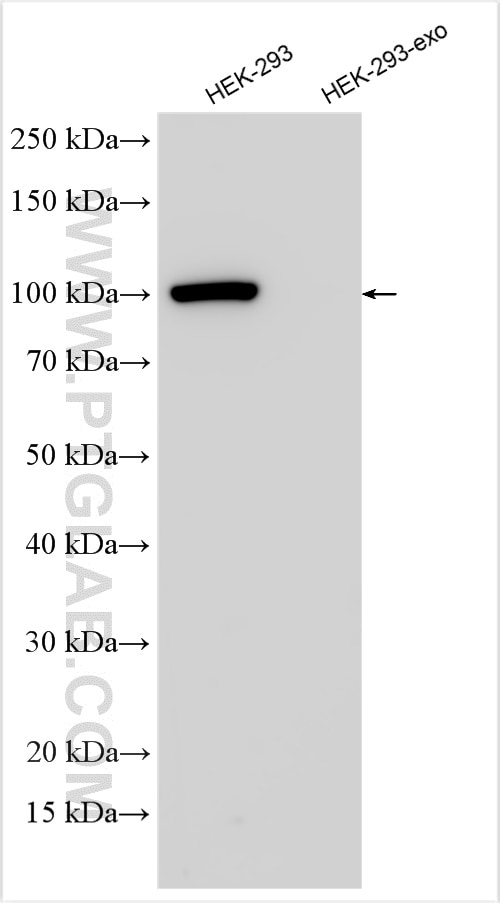
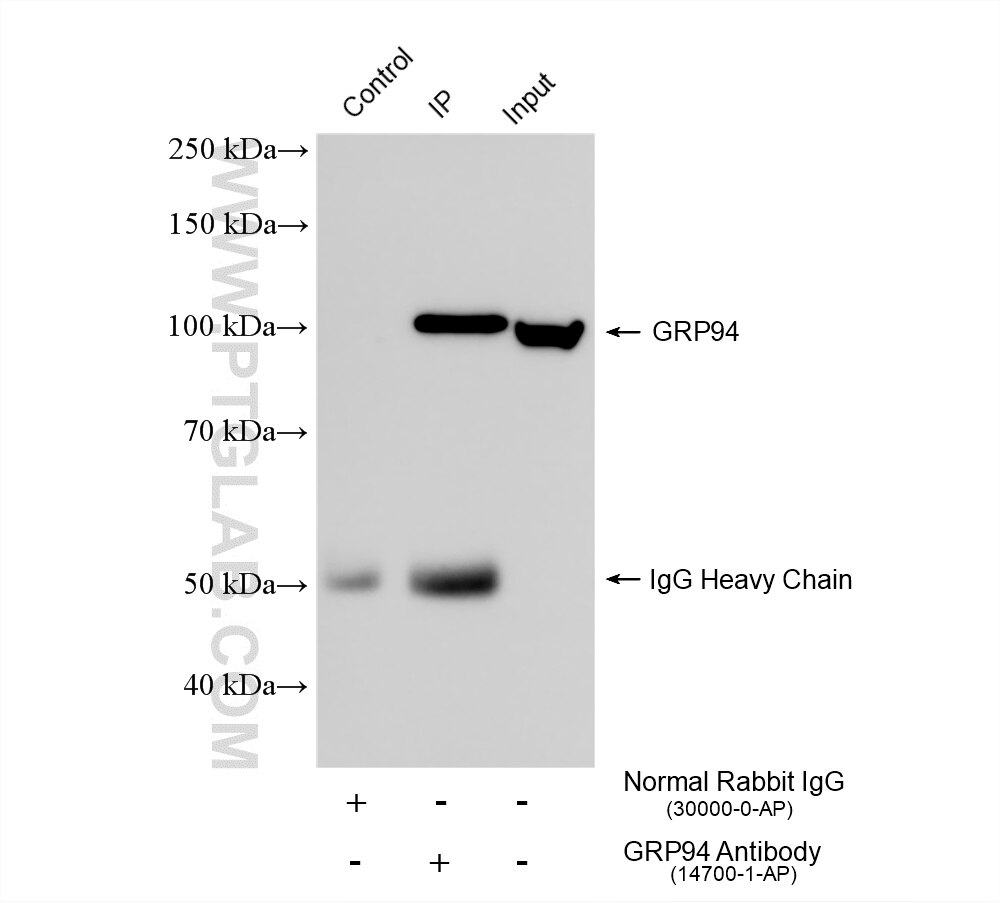
| Positive IP detected in | HEK-293 cells, mouse liver tissue |
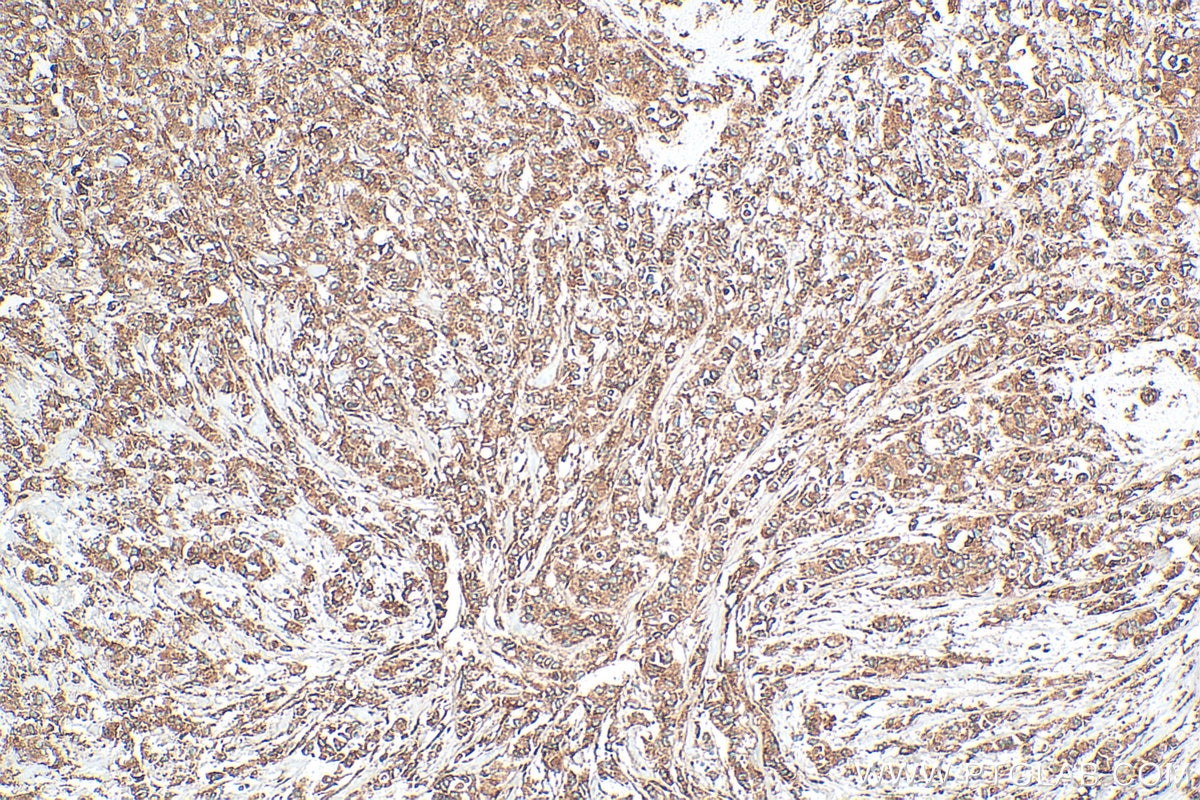
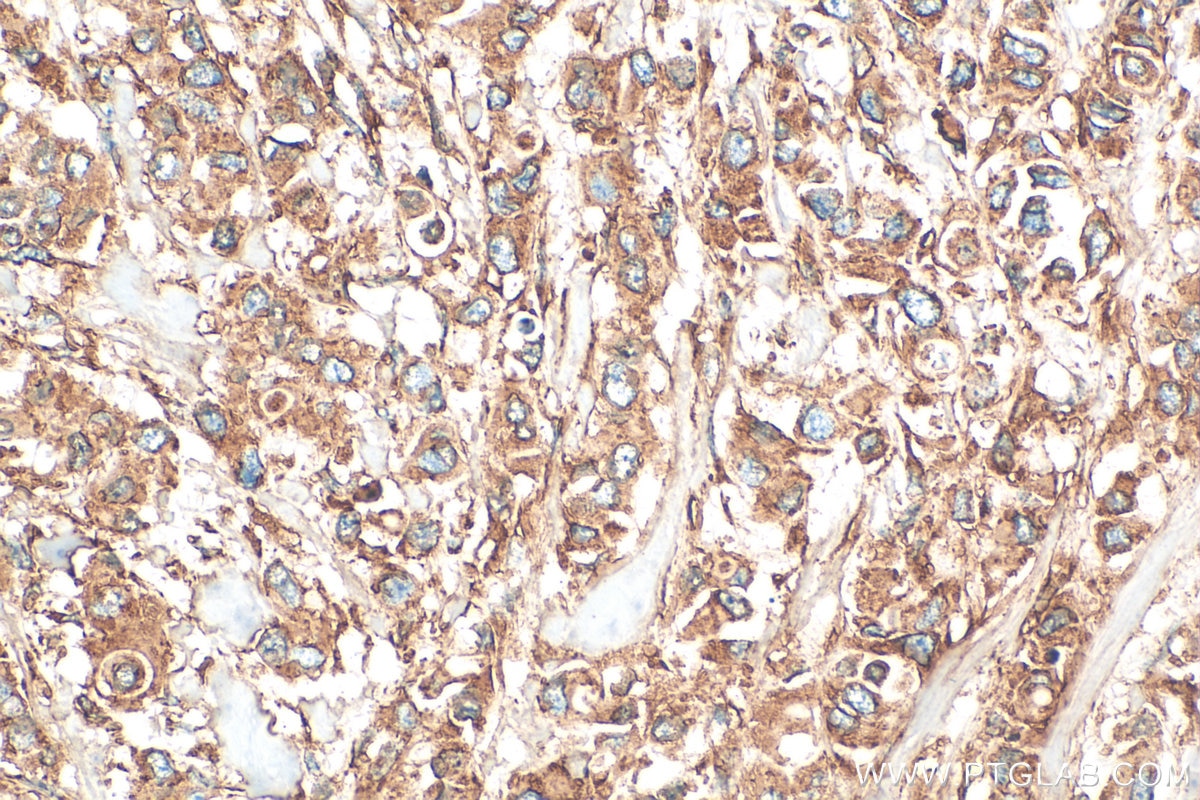
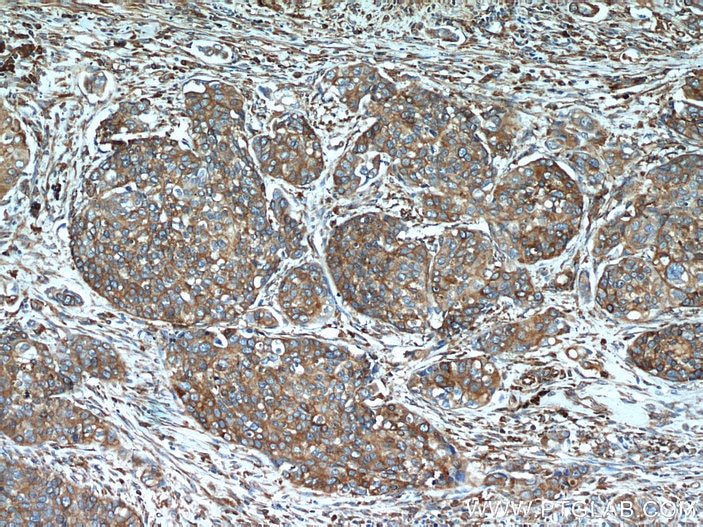
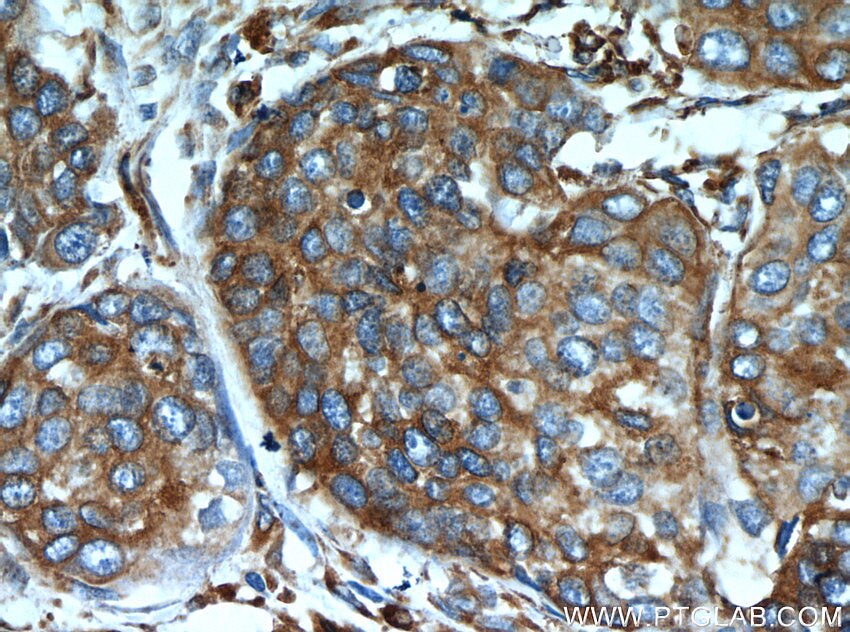
| Positive IHC detected in | human breast cancer tissue, human cervical cancer tissue Note: suggested antigen retrieval with TE buffer pH 9.0; (*) Alternatively, antigen retrieval may be performed with citrate buffer pH 6.0 |
| Positive IF/ICC detected in | U2OS cells, HepG2 cells, HeLa cells |
| Positive FC (Intra) detected in | HeLa cells |
Recommended dilution
| Application | Dilution |
|---|---|
| Western Blot (WB) | WB : 1:2000-1:16000 |
| Immunoprecipitation (IP) | IP : 0.5-4.0 ug for 1.0-3.0 mg of total protein lysate |
| Immunohistochemistry (IHC) | IHC : 1:500-1:2000 |
| Immunofluorescence (IF)/ICC | IF/ICC : 1:50-1:500 |
| Flow Cytometry (FC) (INTRA) | FC (INTRA) : 0.40 ug per 10^6 cells in a 100 µl suspension |
| It is recommended that this reagent should be titrated in each testing system to obtain optimal results. | |
| Sample-dependent, Check data in validation data gallery. | |
Published Applications
| KD/KO | See 2 publications below |
| WB | See 50 publications below |
| IHC | See 6 publications below |
| IF | See 3 publications below |
| CoIP | See 1 publications below |
Product Information
14700-1-AP targets GRP94 in WB, IHC, IF/ICC, FC (Intra), IP, CoIP, ELISA applications and shows reactivity with human, mouse, rat samples.
| Tested Reactivity | human, mouse, rat |
| Cited Reactivity | human, mouse, rat |
| Host / Isotype | Rabbit / IgG |
| Class | Polyclonal |
| Type | Antibody |
| Immunogen |
CatNo: Ag6344 Product name: Recombinant human GRP94 protein Source: e coli.-derived, PET28a Tag: 6*His Domain: 455-803 aa of BC066656 Sequence: KLLKVIRKKLVRKTLDMIKKIADDKYNDTFWKEFGTNIKLGVIEDHSNRTRLAKLLRFQSSHHPTDITSLDQYVERMKEKQDKIYFMAGSSRKEAESSPFVERLLKKGYEVIYLTEPVDEYCIQALPEFDGKRFQNVAKEGVKFDESEKTKESREAVEKEFEPLLNWMKDKALKDKIEKAVVSQRLTESPCALVASQYGWSGNMERIMKAQAYQTGKDISTNYYASQKKTFEINPRHPLIRDMLRRIKEDEDDKTVLDLAVVLFETATLRSGYLLPDTKAYGDRIERMLRLSLNIDPDAKVEEEPEEEPEETAEDTTEDTEQDEDEEMDVGTDEEEETAKESTAEKDEL Predict reactive species |
| Full Name | heat shock protein 90kDa beta (Grp94), member 1 |
| Calculated Molecular Weight | 92 kDa |
| Observed Molecular Weight | 100 kDa |
| GenBank Accession Number | BC066656 |
| Gene Symbol | GRP94 |
| Gene ID (NCBI) | 7184 |
| RRID | AB_2233347 |
| Conjugate | Unconjugated |
| Form | Liquid |
| Purification Method | Antigen affinity purification |
| UNIPROT ID | P14625 |
| Storage Buffer | PBS with 0.02% sodium azide and 50% glycerol, pH 7.3. |
| Storage Conditions | Store at -20°C. Stable for one year after shipment. Aliquoting is unnecessary for -20oC storage. 20ul sizes contain 0.1% BSA. |
Background Information
Glucose-regulated protein 94 (GRP94), also called HSP90B1 and GP96, is an endoplasmic reticulum (ER)-resident member of the heat shock protein 90 (HSP90) family (PMID: 33802964; 24658275). Under ER stress conditions, GRP94 accelerates its function as a molecular chaperone. Client proteins of GRP94 include toll-like receptors (TLRs), glycoprotein (GP) IX subunit, insulin-like growth factors (IGFs), proinsulin, and integrins (PMID: 7913987; 32781621; 22079671). As well as being an ER chaperone, GRP94 can also participate in Calcium regulation and is also a regulator of innate and adaptive immunity (PMID: 33802964; 11584270).
Protocols
| Product Specific Protocols | |
|---|---|
| FC protocol for GRP94 antibody 14700-1-AP | Download protocol |
| IF protocol for GRP94 antibody 14700-1-AP | Download protocol |
| IHC protocol for GRP94 antibody 14700-1-AP | Download protocol |
| IP protocol for GRP94 antibody 14700-1-AP | Download protocol |
| WB protocol for GRP94 antibody 14700-1-AP | Download protocol |
| Standard Protocols | |
|---|---|
| Click here to view our Standard Protocols |
Publications
| Species | Application | Title |
|---|---|---|
Sci Adv Platelet P-selectin initiates cross-presentation and dendritic cell differentiation in blood monocytes. | ||
EMBO J Rab2A-mediated Golgi-lipid droplet interactions support very-low-density lipoprotein secretion in hepatocytes | ||
Theranostics Exosomes from mesenchymal stem cells modulate endoplasmic reticulum stress to protect against nucleus pulposus cell death and ameliorate intervertebral disc degeneration in vivo. | ||
Redox Biol Exogenous spermine attenuates rat diabetic cardiomyopathy via suppressing ROS-p53 mediated downregulation of calcium-sensitive receptor. | ||
Haematologica Comparative analysis of ChAdOx1 nCoV-19 and Ad26.COV2.S SARS-CoV-2 vector vaccines. |