Validation Data Gallery
Product Information
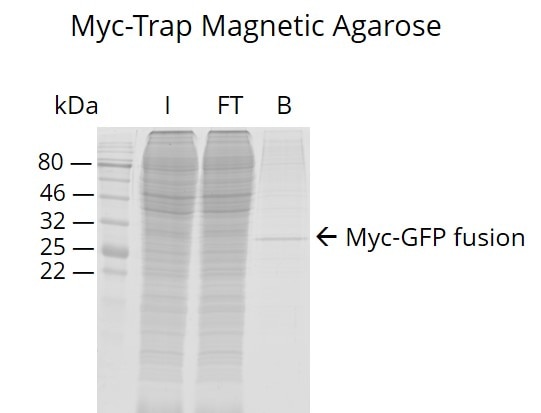
The ChromoTek Myc-Trap® Magnetic Agarose is an affinity resin for IP of Myc-fusion proteins. It consists of a Myc Nanobody/VHH coupled to magnetic agarose beads.
| Description | Immunoprecipitation of Myc-tagged proteins and their interacting factors with anti-Myc Nanobody conjugated to beads.
• Fast, reliable & efficient one-step immunoprecipitation • Ready-to-use • No heavy & light antibody chains • Stable under harsh washing conditions • Suitable for downstream mass spec analysis |
| Applications | IP, CoIP, ChIP, RIP |
| Specificity/Target | Myc-tag sequence motif EQKLISEEDL at the N-terminus, C-terminus, or internal site of the fusion protein. Endogenous c-myc is NOT bound. |
| Binding capacity | 17.5 μg of recombinant Myc-tagged protein (~42 kDa) per 25 μL bead slurry |
| Conjugate | Magnetic agarose beads; bead size: ~40 µm (cross-linked 6 % magnetic agarose beads) |
| Elution buffer | 2x Myc peptide. SDS sample buffer 0.2 M glycine pH 2.5 |
| Wash buffer compatibility | 2 M NaCl, 10 mM DTT, 2 % Nonidet P40 Substitute, 1 % Triton X-100 |
| Type | Nanobody |
| Class | Recombinant |
| Host | Alpaca |
| Affinity (KD) | 1x Myc-tag: Dissociation constant KD of 500 nM 2x Myc-tag: Dissociation constant KD of 0.5 nM |
| Compatibility with mass spectrometry | The Myc-Trap® is optimized for on-bead digestion. For the application note, please click here: On-bead digest protocol for mass spectrometry |
| RRID | AB_2631370 |
| Storage Buffer | 20% ethanol |
| Storage Condition | Shipped at ambient temperature. Upon receipt store at 4°C. Stable for one year. Do not freeze! |
| Size | 25ul/reactions (eg:20rxns=500ul slurry) |
Publications
| Application | Title |
|---|---|
Cell Human-Specific NOTCH2NL Genes Expand Cortical Neurogenesis through Delta/Notch Regulation. | |
Cell Metab Insulin and Leptin/Upd2 Exert Opposing Influences on Synapse Number in Fat-Sensing Neurons. | |
Nat Commun A transcriptional activator effector of Ustilago maydis regulates hyperplasia in maize during pathogen-induced tumor formation | |
Cell Host Microbe CD31 (PECAM-1) Serves as the Endothelial Cell-Specific Receptor of Clostridium perfringens β-Toxin. |
Reviews
The reviews below have been submitted by verified Proteintech customers who received an incentive for providing their feedback.
FH Rupa (Verified Customer) (07-21-2025) | The Myc-trap magnetic agarose beads are very efficient and robust. I used them to immunoprecipitate Myc-tagged protein from T. brucei cell lysate and it gave a clean prep, free of heavy and light chains contamination.
|
FH Amy (Verified Customer) (06-10-2022) | Clean and efficient precipitation of Myc-tagged bait proteins, HEK293T cells transfected with Myc-tagged proteins and lysed in RIPA buffer prior to precipitation with Myc-Trap magnetic agarose beads.
|