Validation Data Gallery
Product Information
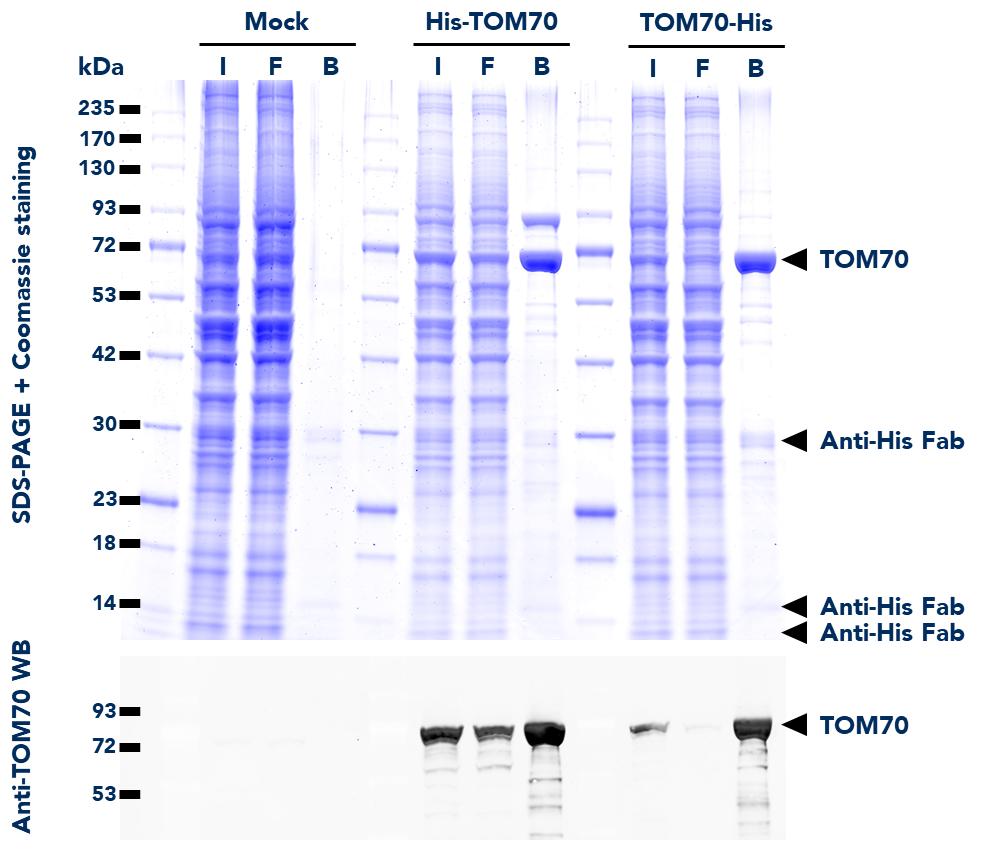
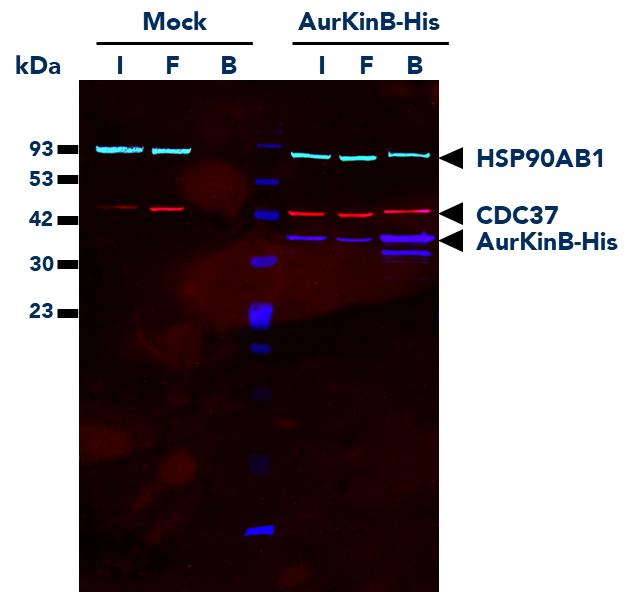
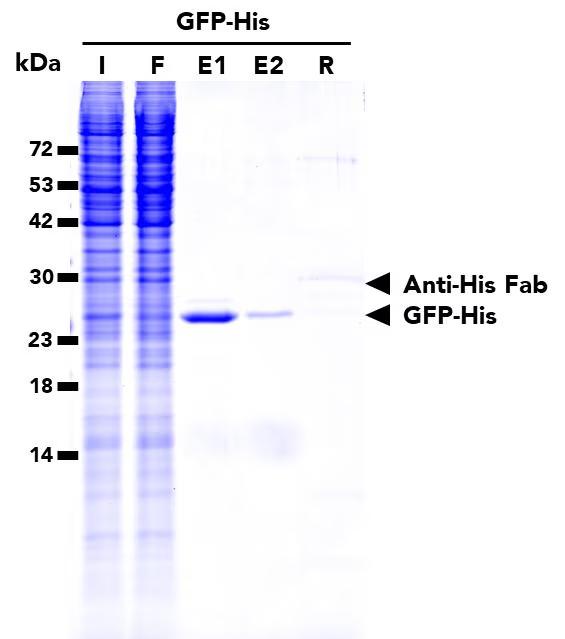
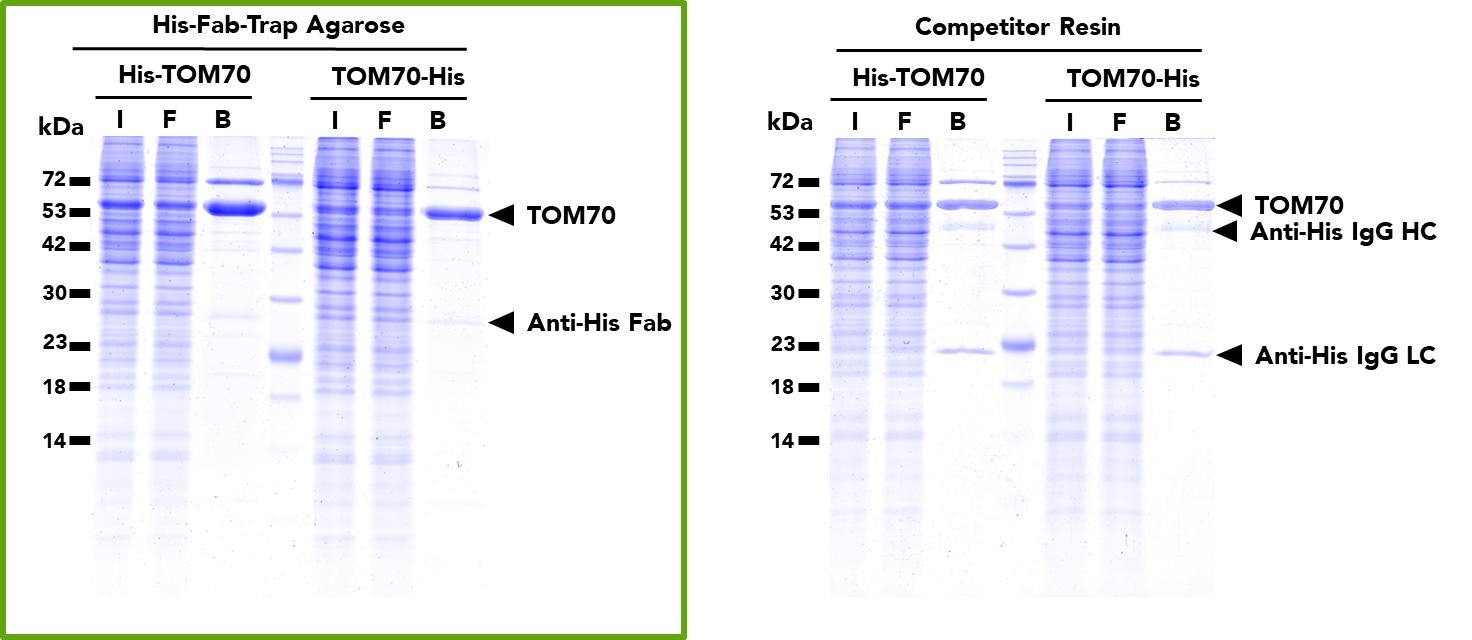
The ChromoTek His Fab-Trap® Agarose consists of an anti-His-tag Fab fragment, which is covalently bound to agarose beads. It can be used for the immunoprecipitation of proteins fused to the His-tag from cell extracts of various organisms.
| Description | Immunoprecipitation of His-tagged proteins with anti-His-tag Fab-fragment conjugated to agarose beads. • Fast, reliable & efficient one-step immunoprecipitation • Ready-to-use • Low background • Stable under harsh washing conditions • Suitable for downstream mass spec analysis • Works in samples from: mammals, plants, yeast, etc. |
| Applications | IP, Co-IP |
| Specificity/Target | Binds specifically to the His-tag (also known as hexahistidine or His6-tag) fused to a protein of interest at N-, C- or internal position. Please note that the affinity is highest for a C-terminal fusion (dissociation constant KD of 10 nM for a C-terminal His-tag and 220 nM for an N-terminal His-tag). |
| Binding Capacity | 25 ug of recombinant His-tagged protein (~30kDa) per 25 uL bead slurry |
| Conjugate | Agarose beads; ~90 um (cross-linked 4% agarose beads) |
| Elution buffer | 2x SDS-sample buffer (Lämmli), 200 mM glycine pH 2.5 or 100 µM His-Peptide |
| Wash Buffer Compatibility | 2 M NaCl, 5 mM DTT, 5 mM β-mercaptoethanol, 5 mM TCEP, 2% NP40, 2% Triton X-100, 0.1% SDS, 5 M Urea |
| Type | Fab-fragment |
| Class | Recombinant |
| Host | Mouse |
| Affinity (KD) | 10 nM for a C-terminal His-tag and 220 nM for a N-terminal His-tag |
| Compatibility with mass spectrometry | The His Fab-Trap® is optimized for on-bead digestion. For the application note, please click here: On-bead digest protocol for mass spectrometry |
| RRID | AB_3661838 |
| Storage Buffer | PBS pH 7.4. Preservative: 0.09% sodium azide |
| Storage Condition | Shipped at ambient temperature. Upon receipt store at +4°C. Stable for one year. Do not freeze! |
| Size | 25ul/reactions (eg:20rxns=500ul slurry) |
Reviews
The reviews below have been submitted by verified Proteintech customers who received an incentive for providing their feedback.
FH Talita (Verified Customer) (09-27-2025) | It was my first time performing the purification using beads, and unfortunately it did not work very well. The protein yield was very low, so I performed the concentration using a centrifuge device. Subsequently, the protein quantification and followed by a western blotting. Unfortunately, the analysis was not successful and I was not able to detect the his-tagged protein. However, I would give this product another try due to its short protocol.
|