Neural stem / neural progenitor cell (NSC/NPC) markers
NSC/NPC markers as a tool for specific neuronal subtypes and identification of glial cells.
Similar to embryonic stem cells (ESCs), induced pluripotent stem cells (iPSCs) can be induced into region-specific neural progenitor cells (NPCs) and eventually differentiated into specific neuronal subtypes and glial cells (1,2).
NPCs are a population of cells with self-renewal and multipotent differentiation ability, meaning they are able to proliferate and maintain the NPC pool as well as subsequently differentiate into regional and spatially distinct neurons and glial cells to execute specific functions. The culture of NSCs in chemically defined neural induction medium results in the differentiation of progenitor cells into different neuronal subtypes.
The proliferative NPCs are characterized by the expression of SRY (sex determining region Y)-box 2 (SOX2) and paired box 6 (KO validated PAX6 antibody, Figure 1).
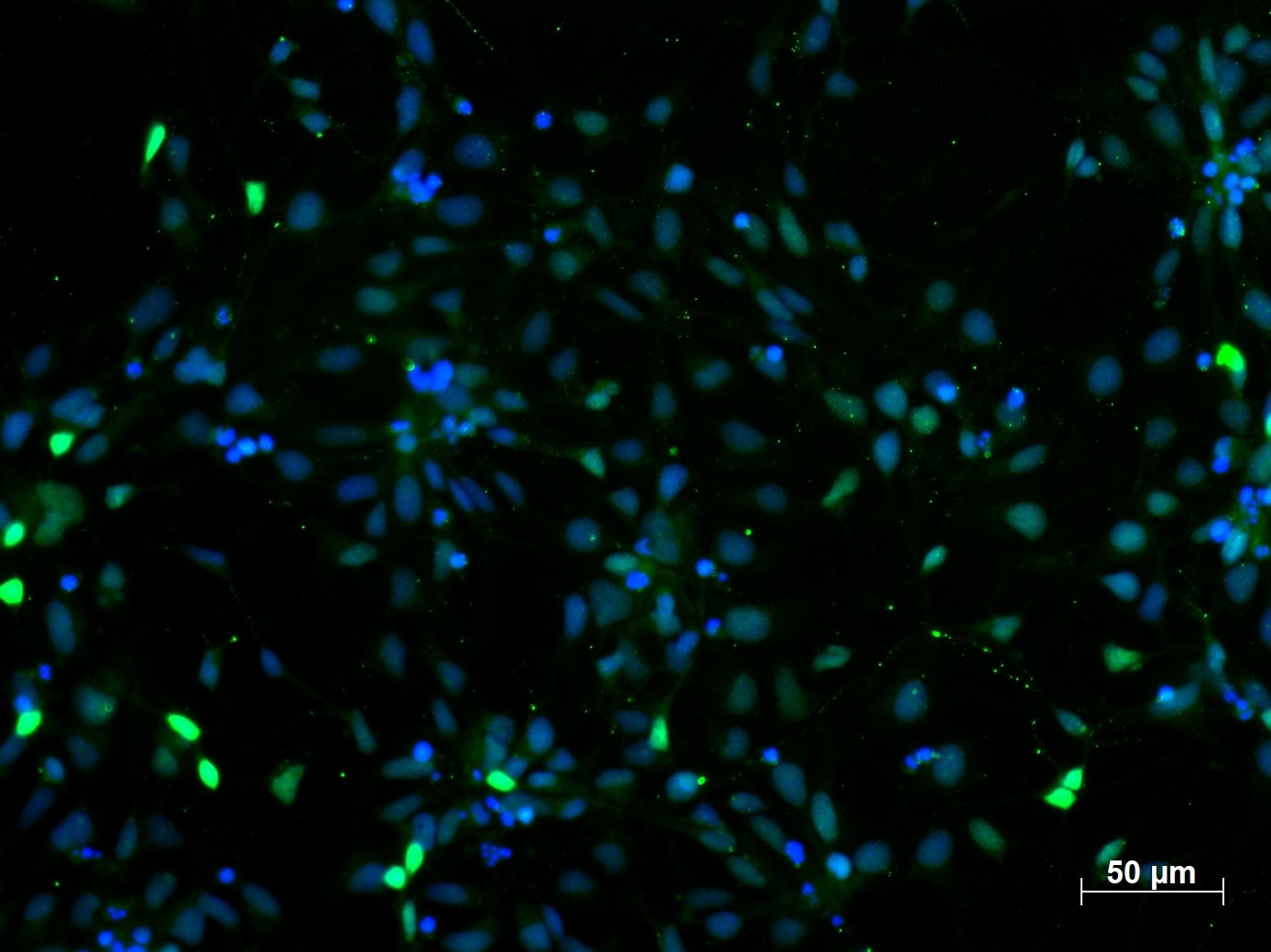
Immunofluorescent staining of PAX6 (12323-1-AP, 1:250 dilution) with 4% PFA fixed control human induced pluripotent stem cells (hiPSC) derived neuronal precursor cells (NPCs). (Green: PAX6; Blue: DAPI). Provided by BioTalentum Ltd., Hungary.
ESC-derived NPCs can be differentiated into three main neural lineages (neurons, astrocytes, and oligodendrocytes). From gestational week 4 to 6, human NPCs prepare a defined number of cells for subsequent neural differentiation with several rounds of symmetrical division. Differentiation of human cortical neurons is initiated at gestational week 6. At this time point the symmetrical division of NPCs is changed to asymmetrical division. Asymmetrical NPC division results in the generation of one NPC and one neuron or glial cell (3).
The efficiency of the neural induction in vitro is usually monitored by NESTIN, PAX6, and SOXs gene expression analysis (4). For terminal differentiation into cortical neurons, the cells are monitored by analysis of Tubulin Beta 3 class III (TUBB3, Figure 2), and microtubule-associated protein 2 (MAP2, Figure 3) expression level.
Self-renewal of NSCs is also dependent on BMI-1 (10832-1-AP and 66161-1-Ig), a member of the polycomb-group proteins, which regulates gene expression by chromatin remodeling. BMI-1 is expressed by NSCs and promotes self-renewal and post-natal maintenance by symmetric divisions, but its loss has no effects on asymmetric divisions and progenitor proliferation.
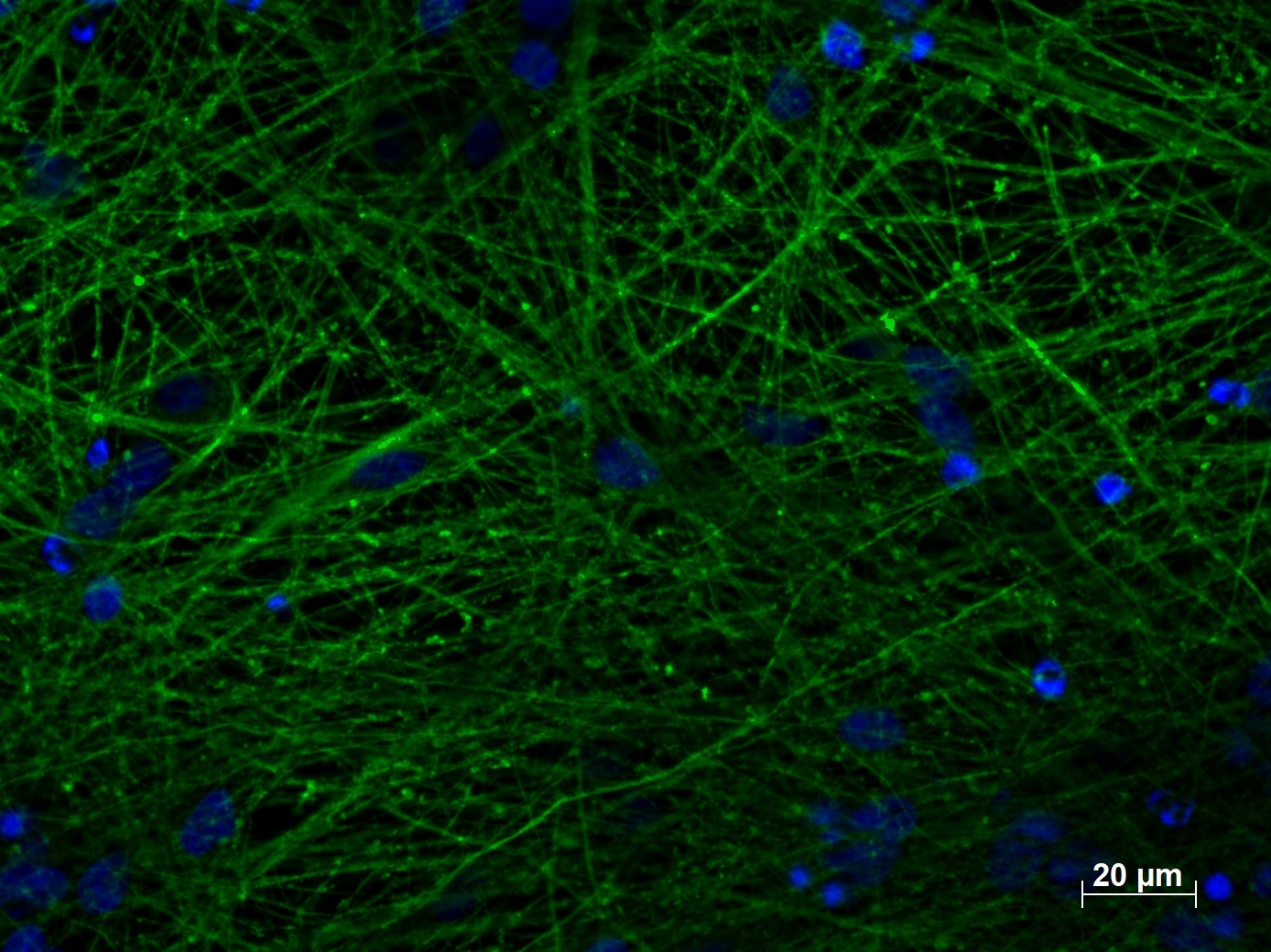
Figure 2. Immunofluorescent staining of TUBB3 (66375-1-lg, 1:250) with 4% PFA fixed control hiPSC-derived neuronal cultures (35 days old). (Green: TUBB3; Blue: DAPI). Provided by BioTalentum Ltd., Hungary.
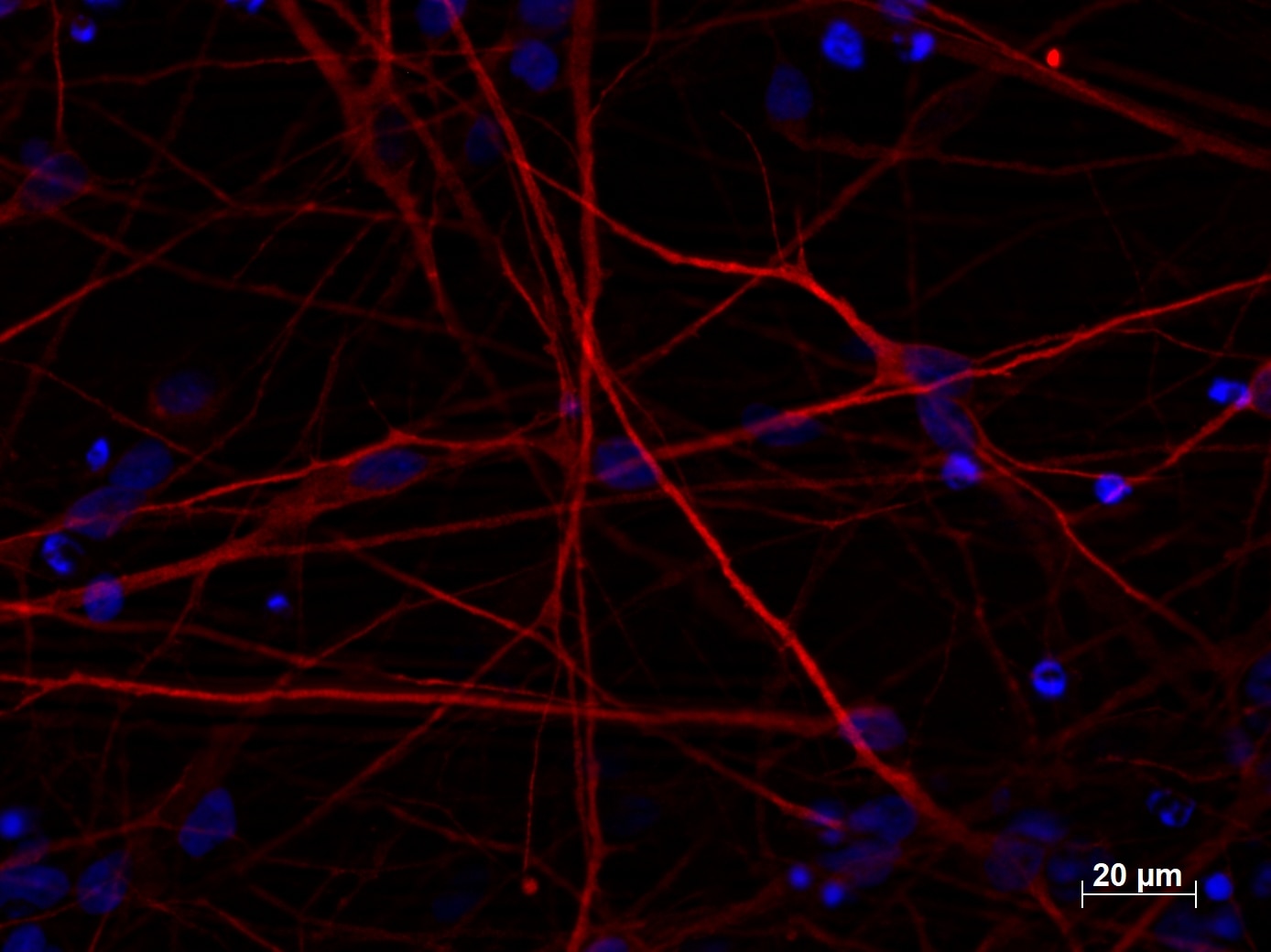
Figure 3. Immunofluorescent staining of MAP2 (17490-1-AP, 1:250 dilution) with 4% PFA fixed control hiPSC-derived neuronal cultures (35 days old). (Red MAP2; Blue: DAPI). Provided by BioTalentum Ltd., Hungary.
The neuronal population from NPCs can be defined using antibodies against neuronal markers (MAP2 and TUBB3) and can be further differentiated into neurons through a monolayer culture system or 3D structures (Figure 4). NPCs provide novel tools for in vitro cellular modeling and mimicking neural development, uncovering the dynamic pathology of neurodegenerative disease, and for the development of potential cell replacement therapies (5, 6).
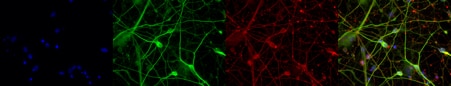
Figure 4. Immunofluorescent staining of MAP2 (17490-1-AP, 1:250 dilution) and TUBB3 (66375-1-lg, 1:250) with 4% PFA fixed control hiPSC-derived neuronal cultures (35 days old). (Red MAP2; Green: TUBB3; Blue: DAPI). Provided by BioTalentum Ltd., Hungary.
References
2) Highly efficient neural conversion of human ES and iPS cells by dual inhibition of SMAD signaling.
3) Asymmetric cell division during neurogenesis in Drosophila and vertebrates.