Ubiquitin Antibody
Ubiquitin and its broad role in cellular machinery.
The importance of ubiquitin and ubiquitin-like proteins (1) in physiology and medicine has evolved due to the accumulation of evidence supporting a major role of these proteins in the regulation of various biological and pathological phenomena.
Ubiquitin (2) is a 76-amino acid protein, which is highly conserved across all eukaryotes. Remarkably, human and yeast ubiquitin share over 95% sequence identity. The key role of ubiquitin in basic cellular processes was cemented with the awarding of the 2004 Nobel Prize in Chemistry to ubiquitin research (3).Ubiquitin (Figure 1) can be attached to substrate proteins as a single moiety or in the form of polymeric chains in which successive ubiquitin molecules are connected through specific isopeptide bonds. Once attached to a substrate, the 76-amino acid ubiquitin is subjected to further modifications, creating a multitude of distinct signals with distinct cellular outcomes, referred to as the 'ubiquitin code' (4).
 |
Figure 1. Immunohistochemical of paraffin-embedded human ovary tumor using 10201-2-AP (ubiquitin antibody) at dilution of 1:50 (under 40x lens). |
Protein ubiquitination (5) is a dynamic multifaceted post-translational modification process that transfers ubiquitin molecules to specific target proteins exclusively on lysine (K) residues. This process occurs through an enzymatic cascade that involves three distinct enzymes, namely ubiquitin-activating (E1), ubiquitin-conjugating (E2), and ubiquitin-ligating (E3) (Figure 2).
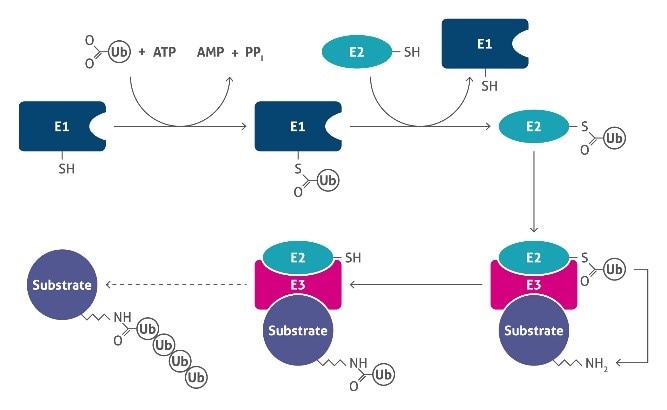 |
Figure 2. Schematic overview of the ubiquitylation system. |
Ubiquitin can be ubiquitinated on seven lysine (Lys) residues or on the N-terminus, leading to polyubiquitin chains that can encompass complex topologies. Alternatively, or in addition, ubiquitin Lys residues can be modified by ubiquitin-like molecules (such as SUMO or NEDD8). Finally, ubiquitin can also be acetylated on Lys, or phosphorylated on Ser, Thr, or Tyr residues, and each modification can dramatically alter the signaling outcome.
The impact of ubiquitination on protein function (6), expression, or localization depends on whether a single ubiquitin moiety (monoubiquitination) or a chain (polyubiquitination) has been attached to a protein (Figure 3). For instance, polyubiquitination through lysines 29 (K29) and 48 (K48) leads to protein degradation via the 26S proteasome, whereas monoubiquitination can lead to endocytosis, DNA repair, or changes in gene expression.
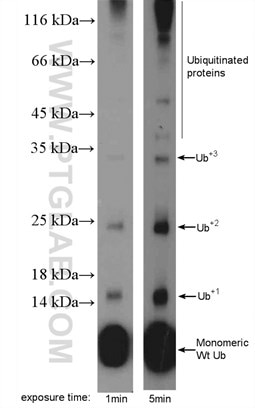 |
Figure 3. MDA-MB-453s cells were subjected to SDS PAGE followed by western blot with 10201-2-AP ( ubiquitin Antibody) at dilution of 1:600. |
Despite the complexity of the ubiquitin system, the progress made in the last decade is astounding and is encouraging that the ubiquitin code can be cracked. Numerous studies have increased our understanding of the role of protein modification via ubiquitin or ubiquitin-like proteins in cancer, metabolic syndromes,neurodegenerative diseases (7), or muscular dystrophies. Ubiquitination is crucial for a plethora of physiological processes, including cell survival and differentiation and innate and adaptive immunity. Recently, ubiquitin has been discovered to play a key role in Mitophagy and (phospho) ubiquitinated mitochondria (8,9).
Related Products
| Antibodies | |
| UBA52 | P62;SQSTM1 |
| UBD | NEDD4 |
| PSMD4 | UBC9 |
Loading control antibodies
| GAPDH Antibody | 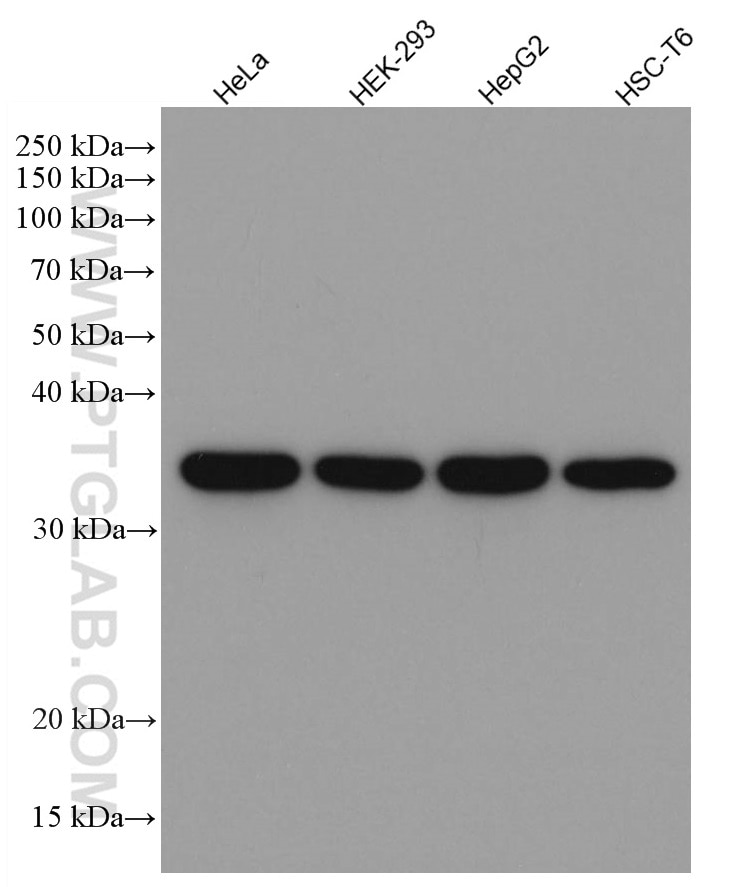 |
| Catalog no.: 60004-1-Ig | |
|
GAPDH is commonly used as a protein loading control in western blot due to its consistently high expression in most cell types. This enzyme participates in several cellular events such as glycolysis, DNA repair, and apoptosis. Proteintech monoclonal GAPDH antibodies are raised against a whole-protein antigen of human origin and have over 4,960 citations. |
| Beta Actin Antibody (KD/KO validated) | 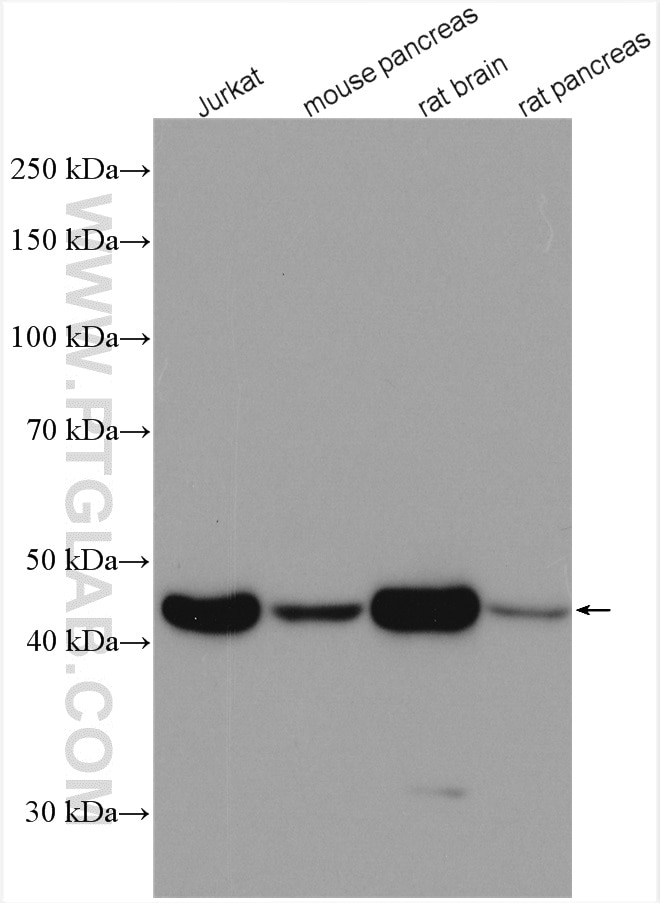 |
| Catalog no.: 66009-1-Ig | |
|
Beta-actin is usually used as a loading control due to its broad and consistent expression across all eukaryotic cell types and the fact that expression levels of this protein are not affected by most experimental treatments. 66009-1-Ig has been cited in over 2,460 publications and has wide species reactivity. |
References
- Ubiquitin modifications.
- The complete amino acid sequence of ubiquitin, an adenylate cyclase stimulating polypeptide probably universal in living cells.
- The Nobel Prize in Chemistry 2004
- The ubiquitin code.
- The ubiquitin system.
- Protein monoubiquitination and polyubiquitination generate structural diversity to control distinct biological processes
- Quantitative proteomics reveal a feedforward mechanism for mitochondrial PARKIN translocation and ubiquitin chain synthesis.
- Mechanisms of mitophagy.
- The ubiquitin kinase PINK1 recruits autophagy receptors to induce mitophagy.