Neuropilin-1 joins ACE2 as SARS-CoV-2 gateway
Landmark paper in Science detailing how Neuropilin-1 is required for SARS-CoV-2 host cell entry, using Proteintech and Chromotek antibodies.
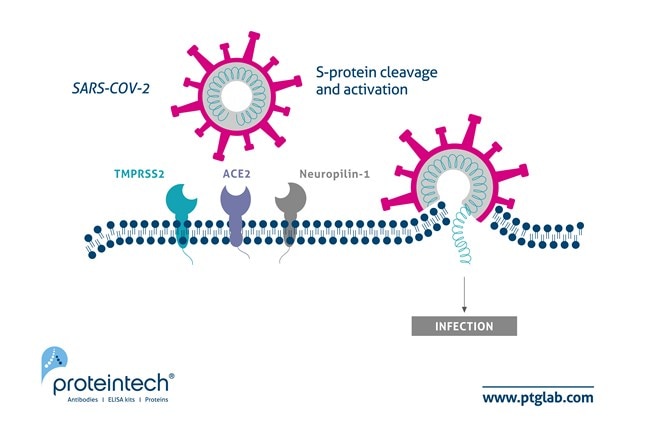
The current coronavirus disease 2019 (COVID-19) pandemic is caused by infection from the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Despite the astounding amount of research on SARS-COV-2, many questions surround the mechanism of viral entry. At this moment, the consensus is that SARS-CoV-2 spike (S) protein first binds to the angiotensin converting enzyme (ACE2) receptor on the host cell membrane. Afterward, the type II transmembrane proteases, TMPRSS2 and HAT, cleave the S-protein, activating it and enabling viral membrane fusion followed by the entry of viral RNA into the host cell. The protease, furin, may also cleave the S-protein into S1 and S2 subunits, increasing infectivity. A landmark paper in Science by Davy et al. described the role of neuropilin-1 as novel mediator in the viral entry mechanism of SARS-CoV-2 (PMID 33082294). They discovered that neuropilin-1, which is expressed abundantly in the respiratory and olfactory systems, binds to furin-cleaved S1 fragments at the cell surface and potentiates host cell entry, thus contributing to the tropism of the virus to these organ systems (Figure 1).
 |
| Figure 1. Updated schematic of SARS-CoV-2 host cell entry to include Neuropilin-1. Image originally adapted from Journal of Virology paper by Heurich et al. PMID: 24227843. |
To test whether neuropilin-1 influenced cell entry, Davy and colleagues assayed SARS-CoV-2 infectivity of cells transfected with different combinations of ACE2, TMPRSS2, and neuropilin-1. Neuropilin-1 expression on its own did not render the cells to infection. However, when expressed in conjunction with ACE2 and TMPRSS2, infectivity was markedly enhanced than with ACE2 and TMPRSS2 alone. Furthermore, when these cells were treated with monoclonal antibodies that functionally blocked the binding of neuropilin-1, infection rates were significantly reduced. The researchers then created a mutant SARS-CoV-2 that had a disrupted furin binding site in its S-protein. The mutant was unable to enhance infection in the co-expressed viral receptor cells, confirming that neuropilin-1 is required to bind furin-cleaved S-protein for its effects. Next, peptides were generated of the C-terminal sequence of the S-protein following furin cleavage and bound to silver nanoparticles (AgNP) for administration in vivo to mouse olfactory epithelia. Six hours after administration, there was a significantly larger uptake of the S-protein peptide AgNPs versus control peptides. Finally, the authors investigated the transcriptomics of bronchial epithelial cells from severely affected COVID-19 patients. RNA expression of neuropilin-1, TMPRSS11a, and furin was significantly amplified in these patients. Interestingly, immunoreactivity of neuropilin-1 was very high in human lung epithelium, versus ACE2, which was hardly detectable, demonstrating the highly pathogenic role of neuropilin-1. This study indicates the importance of neuropilin-1 in facilitating virus-host cell interactions, raising the possibility of a new target for future treatments of COVID-19.
Proteintech’s monoclonal antibody to ACE2 (66699-1-Ig) was used in western blots to quantify the overexpression of ACE2 in HEK-293 cells, for use as an in vitro model of SARS-CoV-2 infection.
ChromoTek’s GFP-Trap® and RFP-Trap® were used in a special Co-IP approach to co-immunoprecipitate different GFP- and mCherry-tagged protein constructs and VSV-Spike pseudotyped virus for quantification on western blot. For example, mCherry-b1 or mCherry-b1 T316R protein was expressed in HEK293T cells and pulled down by RFP-Trap. After enrichment of the mCherry protein constructs on RFP-Trap, VSV-Spike pseudotyped virus was added to the pre-loaded beads to co-immunoprecipitate the virus. Using this set-up, the authors were able to skip the usual purification step with GFP- or Cherry-tagged proteins, and instead captured them from cell lysate on beads and used the proteins to analyze the virus interaction and quantify it.
Information on our COVID-19-related reagents, including ACE2 and Neuropilin-1 antibodies, can be found on our COVID-19 page. More information on ChromoTek’s nanobody-based reagents can be found here.
Related Content
Scientists unravel why Omicron spreads faster
How Nanobodies could advance Sars-CoV diagnostics
Neutralizing antibodies and their role in COVID-19 research
How fluorescent proteins can be applied in SARS-CoV-2 research
Serious about Serology: Understanding COVID19 Antibody Tests
Characterization of COVID-19 antibodies by Bio-Layer Interferometry using Nano-CaptureLigands

Support
Newsletter Signup
Stay up-to-date with our latest news and events. New to Proteintech? Get 10% off your first order when you sign up.