Targeting Purinergic Signaling: A New Frontier in Cancer Immunotherapy
Written by Sepideh Jahangiri, PhD, University of Montreal
Purinergic signaling plays a critical role in regulating immune responses within the tumor microenvironment (TME). This complex system is driven by converting extracellular ATP (eATP) into adenosine (Ado), a transition that profoundly influences immune dynamics in cancer. While eATP acts as a potent immune stimulant, triggering inflammatory responses at high concentrations, its role in solid tumors is more complex (1). Tumor cells, under metabolic or hypoxic stress, release large amounts of ATP into the extracellular space, leading to significantly higher ATP concentrations compared to healthy tissue. To counteract the immune-activating effects of ATP, cancer cells often overexpress ectonucleotidases, such as CD39 and CD73, which rapidly hydrolyze ATP into immunosuppressive Ado (2). This shift enables tumors to evade immune detection and promote survival by reducing inflammation. This dual role of promoting and supressing immune responses makes the purinergic pathway a key target in cancer therapy. Hence, strategies that aim to either boost ATP levels, limit Ado accumulation, or inhibit Ado receptors are being actively explored in immune-oncology (3).
eATP and Its Receptors: A Double-Edged Sword in Cancer
ATP acts as a pleiotropic molecule that can either promote cell proliferation or trigger cell death, depending on its extracellular concentration and the specific P2 receptor expressed on tumor and immune cells. Low levels of eATP are associated with immune suppression and tumor growth, whereas higher concentrations prime inflammatory immune cell infiltration and anti-tumor activity. Tumor-infiltrated immune cells induce macropores in cancer cell membranes, causing membrane depolarization and triggering immunogenic cell death (ICD), which further increases the rate of antigen presentation to the infiltrated immune cells (4).
eATP activates several receptors belonging to the P2X family with different ranges of affinity and desensitization. Members of the P2X family—P2X1R to P2X7R—are ATP-gated non-selective cation channels. They are recognized based on their affinity to ATP and their desensitization kinetics. For example, P2X1R has the highest affinity (nM range) but rapidly desensitizes, whereas P2X7R has the lowest affinity (µM range) and shows little to no desensitization. ATP exerts its anti-tumor immune effects primarily through P2X7R (5).
Three key mechanisms have been proposed to control the downstream signaling effects of P2X7R activation:
-
Pro-inflammatory immune responses: At high ATP concentrations, P2X7R activation triggers the PI3K/AKT and AMPK-PRAS40-mTOR signaling cascades leading to cancer cell death. Prolonged activation recruits the NLRP3 inflammasome complex, resulting in IL-18 and IL-1B secretion and involvement of adaptive inflammatory responses (Figure 1A) (6, 7).
-
Immune Recruitment and Activation: P2X7R interaction with other purinergic receptors (P2X, P2Y, and PANX1 families) on monocytes and dendritic cells (DCs) promotes macrophage chemotaxis and DC activation. In the adaptive immune context, ATP-mediated P2X7R activation drives the activation of both CD4 and CD8 T cells, NK cells, and differentiation of Th17 immune subsets. Additionally, P2X7R suppresses myeloid-derived suppressor cells (MDSCs), inhibits regulatory T cell (Treg) differentiation, and induces Treg apoptosis, shaping the immune response toward an inflamed TME (Figure 1B) (8, 9).
-
Phagocytosis and Antigen Presentation: P2X7R interacts with cytoskeletal proteins, such as NMHC-IIA, to enhance macrophage scavenger and phagocytotic activity. This P2X7R-NMHC-IIA complex is implicated in improving antigen presentation by tumor-infiltrating macrophages, potentially aiding the activation of immune effector T cells (10).
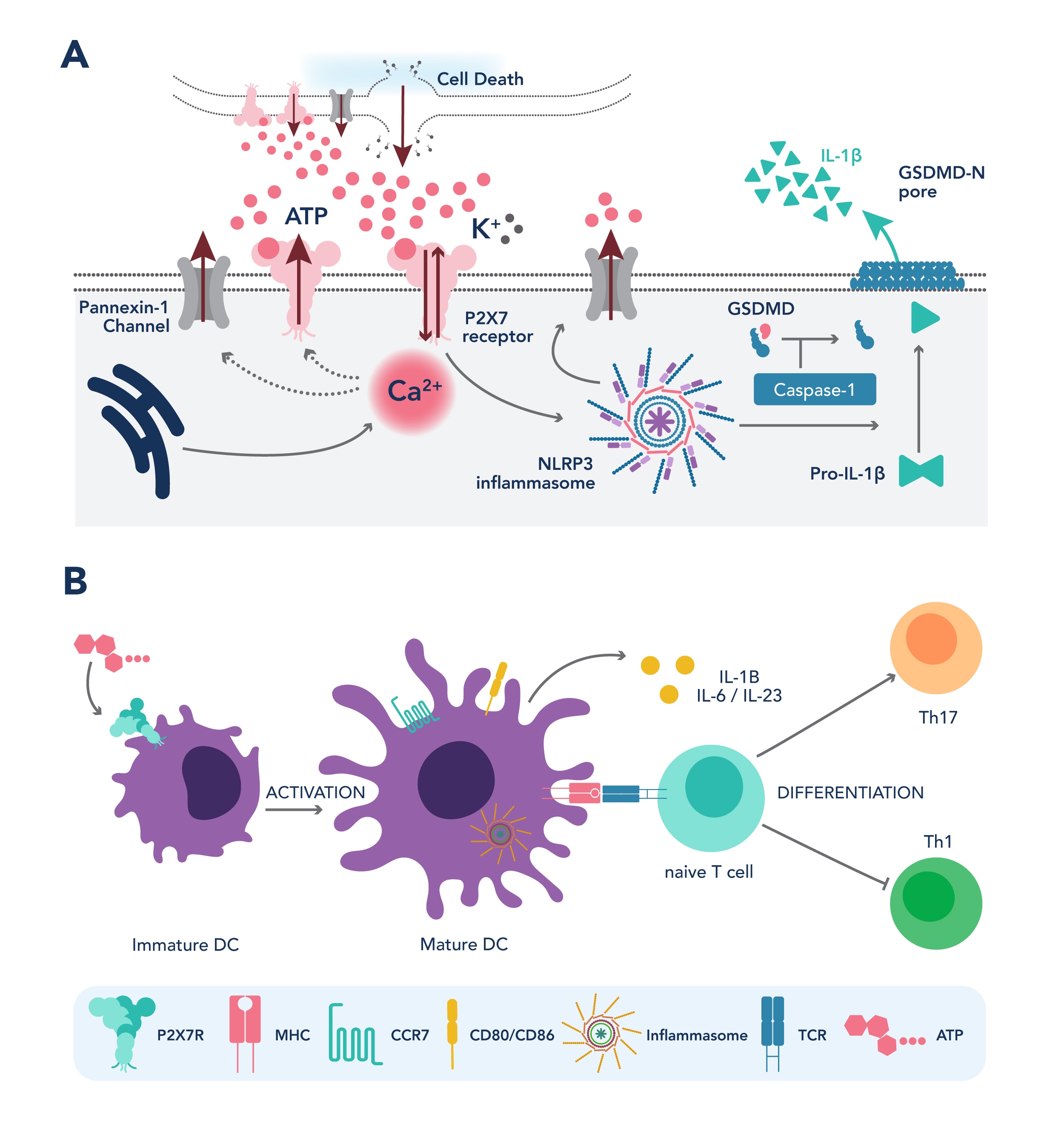
Figure 1. P2X7R induces pro-inflammatory immune response (A) and anti-tumor immune cell activation (B)
Adenosine and cancer immune evasion
Ado exerts its immunosuppressive effects mainly through A2AR and A2BR. Activation of A2AR inhibits CD8-positive T cells and NK cells and promotes the expansion of Treg cells. A2BR activation induces angiogenesis and contributes to immunosuppressive TME (3). Upon A2AR stimulation, adenylyl cyclase (AC) is activated, synthesizing intracellular cAMP. Elevated cAMP—an important secondary messenger—suppresses multiple immune effector cells (T cells, B cells, NK cells, DCs) while enhancing immune suppressor cells (Treg, MDSCs, M2 macrophages) (11). Notably, Tregs not only exploit Ado for immune suppression but also elevates Ado concentration by overexpressing CD39 and CD73, creating a positive feedback loop of immune suppression.
Interestingly, inosine—the product of Ado deaminase (ADA)—also binds to A2AR and further suppresses immune responses via the ERK1/2 pathway. Unlike Ado, inosine has a longer half-life, which persists the immunosuppressive signaling even after Ado levels have declined (11). Elevated Ado concentration is associated with poor prognosis in lung, breast, and melanoma cancers, underscoring the urgency of developing Ado-targeted immunotherapies (3).
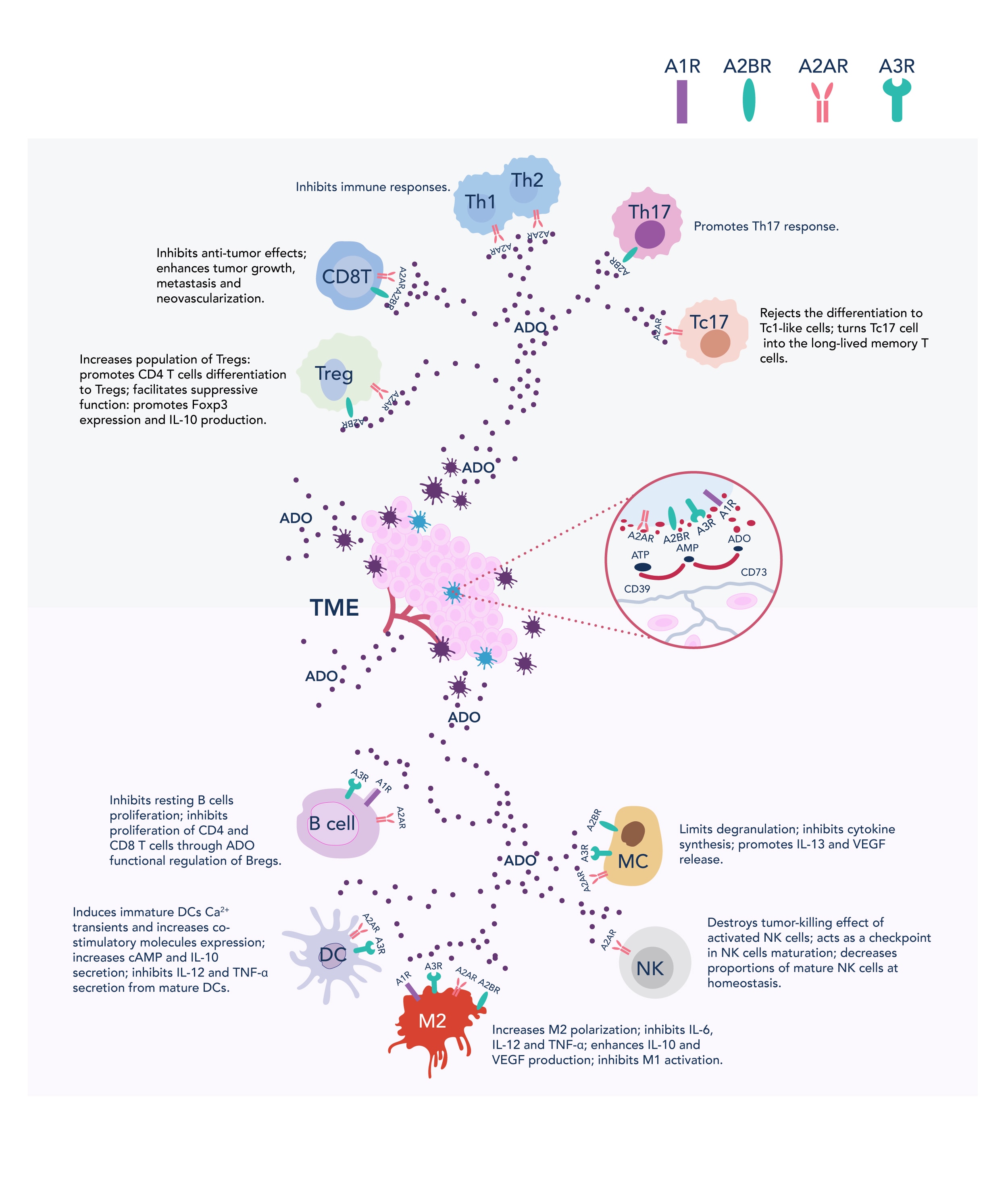 Figure 2. Role of Ado in immune suppression (adapted from https://cancerci.biomedcentral.com/articles/10.1186/s12935-020-01195-x)
Figure 2. Role of Ado in immune suppression (adapted from https://cancerci.biomedcentral.com/articles/10.1186/s12935-020-01195-x)
Therapeutic Opportunities Targeting the Purinergic Pathway
Current therapeutic approaches focus on:
-
Enhancing eATP release using external modalities such as focused ultrasound to promote TME inflammation (12).
-
Inhibiting CD39 and CD73 to prevent Ado production (2).
-
Blocking A2AR and A2BR to neutralize Ado’s downstream effects (13, 14).
-
Combination of the purinergic pathway with immune checkpoint inhibitors (ICI) to overcome resistance to ICI therapy (11, 15-17).
CD39 inhibitors (e.g., IPH-5201) or CD73 inhibitors (IPH5301, CPI-006, and oleclumab/MEDI9447) show encouraging immune activation and synergy with ICIs. Similarly, A2AR antagonists like CPI-444 (ciforadenant) and AZD4635 demonstrate efficacy in preclinical models and early-phase clinical trials, especially when combined with PD-1 or CTLA-4 inhibitors (15-17).
Combination therapy is a particularly promising avenue given Ado’s role in ICI resistance. Dual targeting of Ado signaling and checkpoint pathways may reinvigorate anti-tumor immunity.
Additionally, clinical trials of Ado pathway inhibitors are yielding promising results. For instance:
-
CPI-444 + Atezolizumab (aPD-1) showed clinical activity in renal cell carcinoma and NSCLC (18).
-
Oleclumab + Durvalumab (aPD-L1) demonstrated signs of immune activation and tumor response (19).
Despite these successes, challenges remain. Ado receptors are also expressed on non-immune cells, including cardiovascular tissues, raising concerns about off-target effects. Moreover, patient responses can vary based on tumor type, Ado receptor expression, and the immune landscape of the TME. Precision targeting and biomarker-based selection will be key to maximizing therapeutic benefit while minimizing adverse effects.
Future of Adenosinergic Pathway Research in Cancer Immunotherapy
New avenues in targeting adenosinergic pathways are emerging to enhance treatment efficacy. One exciting area of exploration is the development of more potent and selective Ado inhibitors, particularly targeting A2AR and A2BR. These inhibitors, when used in combination with other immunotherapies such as ICIs, are showing potential in amplifying anti-tumor immune responses by overcoming the immunosuppressive environment driven by Ado. Moreover, targeting Ado receptors, either through pharmacologic or genetic modification in CAR-T cells, reveals promising findings with synergistic effects that boost immune activation.
As the complexity of tumor biology and the TME becomes better understood, personalized medicine is gaining importance in targeting the adenosinergic pathway. Individual cancer subtypes may respond differently to Ado modulation, and tailoring therapies based on specific biomarkers or the Ado receptor expression profile in each patient’s tumor could significantly improve outcomes. This personalized approach ensures that patients with high Ado activity benefit the most from Ado-targeting treatments, thus ushering in a more refined and effective era of cancer immunotherapy.
![]() Conclusion: The Road Ahead for Adenosine Modulation in Cancer Therapy
Conclusion: The Road Ahead for Adenosine Modulation in Cancer Therapy
Continued research and clinical trials remain essential to fully understand the complexity of purinergic modulation in cancer immunotherapy. While promising results from early studies have demonstrated the efficacy of CD39, CD73, A2AR, and A2BR antagonists, challenges such as resistance mechanisms and variability in patient responses highlight the need for deeper investigation. Expanding our knowledge of Ado signaling across different cancer types and immune landscapes will be crucial for the development of more effective treatments.
Looking ahead, the potential of Ado-targeted therapies to revolutionize cancer immunotherapy is significant. By integrating these therapies with existing treatments, including ICIs and personalized approaches, there is an opportunity to create more powerful, durable immune responses against tumors. With continued innovation and research, Ado modulation could become a cornerstone in the future of cancer immunotherapy, offering hope for better outcomes and long-term remission for patients across various cancer types.
With 2/3 of the human proteome covered, Proteintech offers a wide selection of products to study purinergic signaling in immunotherapy.
Click on the targets below to explore our offerings:
|
|
References
- Lara R, Adinolfi E, Harwood CA, Philpott M, Barden JA, Di Virgilio F, McNulty, S. P2X7 in Cancer: From Molecular Mechanisms to Therapeutics. Frontiers in Pharmacology. 2020;11:793.
- Allard B, Longhi MS, Robson SC, Stagg J. The ectonucleotidases CD 39 and CD 73: Novel checkpoint inhibitor targets. Immunological Reviews. 2017;276(1):121-44.
- Allard B, Allard D, Buisseret L, Stagg J. The adenosine pathway in immuno-oncology. Nature Reviews Clinical Oncology. 2020;17(10):611-29.
- Di Virgilio F, Adinolfi E. Extracellular purines, purinergic receptors and tumor growth. Oncogene. 2017;36(3):293-303.
- Di Virgilio F, Sarti AC, Falzoni S, De Marchi E, Adinolfi E. Extracellular ATP and P2 purinergic signalling in the tumour microenvironment. Nature Reviews Cancer. 2018;18(10):601-18.
- Li X-Y, Moesta AK, Xiao C, Nakamura K, Casey M, Zhang H, et al. Targeting CD39 in Cancer Reveals an Extracellular ATP- and Inflammasome-Driven Tumor Immunity. Cancer Discovery. 2019;9(12):1754-73.
- Bian S, Sun X, Bai A, Zhang C, Li L, Enjyoji K, et al. P2X7 integrates PI3K/AKT and AMPK-PRAS40-mTOR signaling pathways to mediate tumor cell death. PLoS One. 2013;8(4):e60184.
- Di Virgilio F, Dal Ben D, Sarti AC, Giuliani AL, Falzoni S. The P2X7 Receptor in Infection and Inflammation. Immunity. 2017;47(1):15-31.
- Yip L, Woehrle T, Corriden R, Hirsh M, Chen Y, Inoue Y, et al. Autocrine regulation of T-cell activation by ATP release and P2X7 receptors. The FASEB Journal. 2009;23(6):1685-93.
- Gu BJ, Rathsam C, Stokes L, McGeachie AB, Wiley JS. Extracellular ATP dissociates nonmuscle myosin from P2X(7) complex: this dissociation regulates P2X(7) pore formation. American Journal of Physiology Cell Physiology. 2009;297(2):C430-9.
- Beavis PA, Milenkovski N, Henderson MA, John LB, Allard B, Loi S, et al. Adenosine Receptor 2A Blockade Increases the Efficacy of Anti-PD-1 through Enhanced Antitumor T-cell Responses. Cancer Immunology Research. 2015;3(5):506-17.
- Jahangiri S, Bourdages S, Skora E, Stagg J, Yu F. ATP released by ultrasound targeted microbubble cavitation induces vascular inflammation and improves immune checkpoint blockade efficacy. Theranostics. 2025;15(11):5220-37.
- Leone RD, Lo Y-C, Powell JD. A2aR antagonists: Next generation checkpoint blockade for cancer immunotherapy. Computational and Structural Biotechnology Journal. 2015;13:265-72.
- Yi Y, Zhou Y, Chu X, Zheng X, Fei D, Lei J, et al. Blockade of Adenosine A2b Receptor Reduces Tumor Growth and Migration in Renal Cell Carcinoma. Journal of Cancer. 2020;11(2):421-31.
- Allard B, Pommey S, Smyth MJ, Stagg J. Targeting CD73 Enhances the Antitumor Activity of Anti-PD-1 and Anti-CTLA-4 mAbs. Clinical Cancer Research. 2013;19(20):5626-35.
- Hay CM, Sult E, Huang Q, Mulgrew K, Fuhrmann SR, McGlinchey KA, et al. Targeting CD73 in the tumor microenvironment with MEDI9447. Oncoimmunology. 2016;5(8):e1208875.
- Perrot I, Michaud H-A, Giraudon-Paoli M, Augier S, Docquier A, Gros L, et al. Blocking Antibodies Targeting the CD39/CD73 Immunosuppressive Pathway Unleash Immune Responses in Combination Cancer Therapies. Cell Reports. 2019;27(8):2411-25. e9.
- Fong L, Forde PM, Powderly JD, Goldman JW, Nemunaitis JJ, Luke JJ, et al. Safety and clinical activity of adenosine A2a receptor (A2aR) antagonist, CPI-444, in anti-PD1/PDL1 treatment-refractory renal cell (RCC) and non-small cell lung cancer (NSCLC) patients. Journal of Clinical Oncology. 2017;35(15).
- Herbst RS, Majem M, Barlesi F, Carcereny E, Chu Q, Monnet I, et al. COAST: An Open-Label, Phase II, Multidrug Platform Study of Durvalumab Alone or in Combination With Oleclumab or Monalizumab in Patients With Unresectable, Stage III Non-Small-Cell Lung Cancer. Journal of Clinical Oncology. 2022;40(29):3383-93.
Related Content
Immune Checkpoint Inhibitor Therapy: Revolutionizing Cancer Treatment | Proteintech Group
Cuproptosis in Health and Disease: Copper’s Double-Edged Sword | Proteintech Group
m5C Modifications in RNA and Cancer | Proteintech Group
The Role of Autophagy in Cancer
Support
Newsletter Signup
Stay up-to-date with our latest news and events. New to Proteintech? Get 10% off your first order when you sign up.

