B cell mitochondria: much more than a powerhouse
Nature Immunology paper from a team the University of Oxford detail how mitochondria are essential for B cell development and lymphomagenesis.
Summary of the paper: Yazicioglu, Yavuz F., et al. “Dynamic mitochondrial transcription and translation in B cells control germinal center entry and lymphomagenesis.” Nature Immunology (2023): 1-16.
By Amanda Balboa Ramilo, PhD candidate at Uppsala University.
B lymphocytes (or B cells) are essential components of the adaptive immune response, producing high-affinity antibodies against perceived pathogens. B cells develop from hematopoietic stem cell precursors, differentiating and maturing into different intermediates (pro-B cell, pre-B cell, and immature B cell) in the adult bone marrow. B cells then enter germinal centers where, in the dark zone, they undergo somatic hypermutation of their B cell receptors. Next, B cells move to the light zone where b cells with receptors that have high affinity for the antigen presented by follicular T helper cells are selected for further development into B cell clones (plasma and memory B cells). This is called the germinal center reaction. This process has various oncogenic implications due to the rapidly mutating immunoglobulin gene loci. Thus, germinal center B cells are the origin of most diffuse large C cell lymphomas (the most common non-Hodgkin lymphoma).
B cells in the germinal center proliferate rapidly and are mostly dependent on oxidative phosphorylation, a metabolic process also present in diffuse large B cell lymphomas. In vitro, it has been shown that mitochondria can regulate B cell signaling. However, it is not clear if this is true in vivo. In other cell types, such as T cells, fibroblasts, and myeloid cells, regulation of mitochondrial transcription and translation by Transcription Factor A, Mitochondrial (TFAM) is highly important for cell function. A recent publication by Yazicioglu et al. at the Kennedy Institute of Rheumatology, The University of Oxford (2023, Nature Immunology, PMC10232359) aimed to elucidate the role of TFAM and mitochondrial transcription and translation in the maturation and entry of B cells into the germinal center reaction. Below we present a summary of this exciting paper:
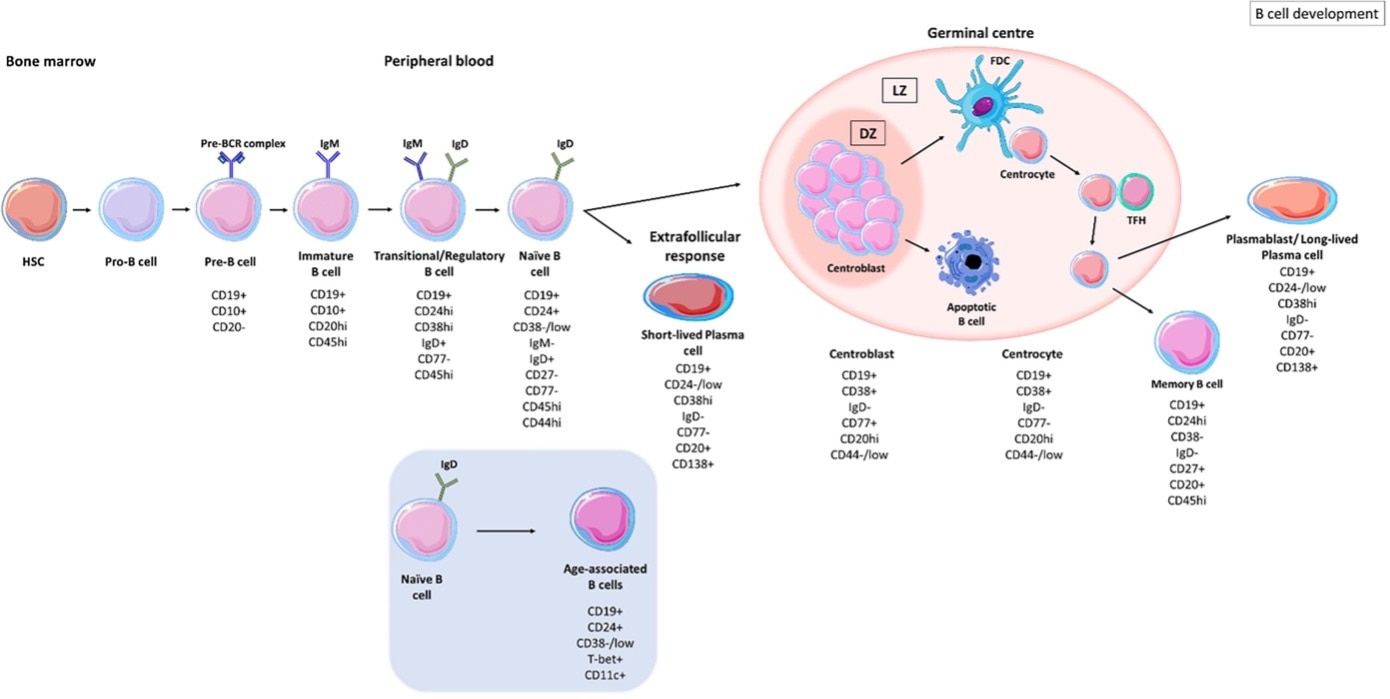
Figure 1: Stages of B-cell development. Figure from Patel et al,. 2021, Frontiers of Immunology (Open source, PMID: 34630404). Key: HSC = hematopoetic stem cell, SMH = somatic hyper mutation, DZ = dark zone, LZ = light zone, FDC = follicular dendritic cell, TFH = T follicular helper cells.
Germinal center B cell mitochondria are highly dynamic organelles, undergoing profound structural changes
Mitochondrial structure and density in germinal center B cells was analyzed by confocal microscopy and flow cytometry, revealing the presence of more numerous and larger mitochondria in B cells committed to the germinal center reaction, compared with naïve B cells present in the surrounding B cell follicle. Not only did these cells have larger mitochondrial mass, but they also presented higher levels of mitochondrial translation and transcription. TFAM was identified in mitochondrial nucleoids, colocalizing with areas of high mitochondrial translation and transcription.
These results suggest that, for B cells to enter the germinal center reaction, they must increase their mitochondrial mass, which they achieve by increasing mitochondrial translation and transcription rates likely stimulated by TFAM.
TFAM is dynamically regulated in B cells and is required for their development and entry into the germinal center reaction, by modulating cellular motility
To assess the relevance of TFAM for normal B cell development, conditional deletion of TFAM in mouse B cells (B-TFAM) was performed. Flow cytometry analysis showed that 1) in the bone marrow, B cells without TFAM failed to progress from the pro-B to the pre-B stage, and 2) in the spleen, there was a profound reduction in the total number of marginal and follicular B cells. Analysis of protein expression by high dimensional flow cytometry showed that the absence of TFAM results in the downregulation of the mitochondrial-encoded COXI and a compensatory upregulation of nuclear-encoded proteins COXIV, ATP5A1, and HSP60. For detection of these proteins, Proteintech’s Coralite conjugated antibodies were used: CoraLite Plus 488-conjugated COXIV Monoclonal antibody (CL488-60251); CoraLite 555-conjugated ATP5A1 Monoclonal antibody (CL555-66037), and CoraLite 594-conjugated HSP60 Monoclonal antibody (CL594-66041).
Specific deletion of TFAM from germinal center B cells (Aicda-TFAM) showed that, after immunization, there were fewer and smaller germinal centers formed with highly disorganized architecture and poor cell compartmentalization.
Single-cell gene expression profiling of immunized Aicda-TFAM cells shows that cells lacking TFAM expression have a dysregulated mitochondrial gene expression, with the cell motility-associated genes being the most affected. This transcriptional profile is suggestive of altered cell trafficking and cytoskeleton dynamics.
Based on these results, the authors suggest that TFAM is essential for normal B cell development and entry into the germinal center. In the absence of TFAM, B cells do not achieve their final mature state and there is a disturbance in mitochondrial protein expression. Furthermore, B cells are less capable of forming organized and functional germinal centers after immunization. Thus, TFAM is essential for maintaining B cells’ cytoskeleton, allowing mobility and spatial positioning.
TFAM is essential for the development of lymphoma. Pharmacological inhibition of mitochondria transcription and translation in human lymphoma cells is a potential treatment target for human disease.
Diffuse large B cell lymphomas during the process of somatic hypermutation are commonly caused by the translocation of MYC to immunoglobulin gene loci. Given that TFAM regulates B cell entry into the germinal center reaction, where somatic hypermutation occurs, the authors hypothesized that deleting TFAM would prevent lymphomagenesis. In a mouse model of lymphoma, the authors demonstrated that deleting TFAM from B cells effectively prevented lymphoma development in mice. In addition, they showed that lymphoma cells express TFAM and have higher levels of mitochondrial translation and transcription. Pharmacological inhibition of mitochondrial transcription and translation thus emerges as a potential therapeutic target in human lymphoma. To test this hypothesis, the authors showed that inhibiting mitochondrial transcription and translation in a human lymphoma cell line reduced cell growth.
To conclude, the study by Yazicioglu et al. established the relevance of Transcription Factor A, Mitochondrial (TFAM) as a regulator of B cell development by promoting the necessary increase in mitochondrial activity and maintenance of the cytoskeleton composition in B cells. This appears to enable the correct formation, function, and output of the germinal center reaction. By identifying TFAM as an important factor in B cell development, this paper also opens the door to studying its role in lymphomagenesis, suggesting new therapeutic targets to explore.
Proteintech products cited:
- CoraLite Plus 488-conjugated COXIV Monoclonal antibody (CL488-60251)
- CoraLite 555-conjugated ATP5A1 Monoclonal antibody (CL555-66037)
- CoraLite 594-conjugated HSP60 Monoclonal antibody (CL594-66041)
Discover our products for flow cytometry here.
Related Content
Flow Cytometry Gating for Beginners
Mitochondrial Respiratory Supercomplexes: A focus on Cox7A isoforms
Want to upgrade your immunofluorescence workflow? Go Direct!
Support
Newsletter Signup
Stay up-to-date with our latest news and events. New to Proteintech? Get 10% off your first order when you sign up.

