GFP-Trap for immunoprecipitation
The GFP-Trap® belongs to ChromoTek’s Nano-Trap family of ready-to-use pull-down reagents. GFP-Trap is the benchmarking reagent for one-step immunoprecipitation (IP) of GFP-fusion proteins. The GFP-Trap consists of an anti-GFP Nanobody coupled to beads. The GFP-Trap provides a high level of IP performance. Next to IP, GFP-Trap can be applied in Co-IP, Co-IP/MS, on-bead assays, and ChIP/RIP analysis.
Contents:
- No heavy and light chain antibody fragments & superior background
- Complete pull-down of GFP-tagged proteins with GFP-Trap
- Fast immunoprecipitation (I)
- Fast immunoprecipitation (II)
- GFP-Trap for a higher level of performance in various applications
- Stringent washing
- Wash buffer compatibility
- Ready-to-use GFP-Trap formats
- IP of fusion proteins expressed at low levels
- Co-IP for Mass Spectrometry (MS) analysis
- Immunoprecipitation of large proteins/protein complexes
- Validation, characterization, and reliability
- Specificity of GFP-Trap
- What is a Nanobody? What is the GFP-Trap?
- Watch the video why GFP-Trap gives the best results for immunoprecipitation (IP)
No heavy and light chain antibody fragments & superior background
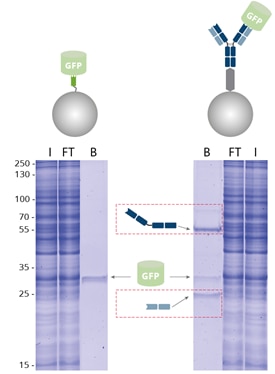 |
| Immunoprecipitation of GFP by GFP-Trap compared with a conventional anti-GFP antibody coupled to Protein A/G beads analyzed by SDS-PAGE. I: Input, FT: Flow-Through, B: Bound. |
Benefits of GFP-Trap:
- No heavy & light antibody chains
- More effective immunoprecipitation
- Less background
When using GFP-Trap for pull-down of GFP-fusion proteins, the amount of immunoprecipitated GFP is significantly higher and the background is reduced, in contrast to an IP conducted with conventional anti-GFP antibody conjugated to Protein A/G beads. Here, the heavy and light antibody chains (dashed, red boxes) are contaminating. GFP-Trap, however, provides pure fractions of immunoprecipitated GFP-fusion protein without contamination of heavy & light antibody chains.
Complete pull-down of GFP-tagged proteins with GFP-Trap
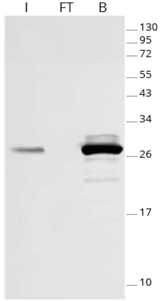 |
| Effectiveness of GFP immunoprecipitation with GFP-Trap: No GFP-fusion protein is detectable in the flow-through fraction by Western blotting, indicating that the GFP-IP was complete because of GFP-Trap’s high binding affinity KD= 1 pM. I: Input, FT: Flow-Through, B: Bound. |
GFP-Trap effectively immunoprecipitates GFP-fusion proteins. Because of GFP-Trap’s high binding affinity with a dissociation constant KD= 1 pM, all GFP-fusion protein is enriched in the bound fraction and no GFP-fusion protein is detectable in the flow-through fraction by Western blotting, indicating a complete pull-down.
Fast immunoprecipitation (I)
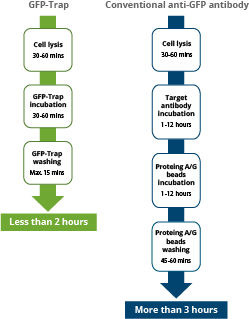 |
| Comparison of the duration of immunoprecipitation using GFP-Trap reagents vs. a conventional anti-GFP antibody, saving significant experimental time of more than 1 hr. |
The GFP-Trap is a ready-to-use reagent that consists of a GFP Nanobody conjugated to beads. Hence, it does not need the additional incubation step required when using Protein A/G beads and conventional antibodies for immunoprecipitation. Experimental and hands-on times are significantly reduced.
Fast immunoprecipitation (II)
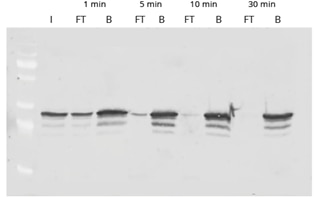 |
| Western Blot of GFP-IP using GFP-Trap. Input (I) plus flow-through (FT) and bound (B) fractions shown after incubation times of 1, 5, 10, and 30 minutes. The disappearance of the FT lane indicates that the pull-down of GFP is completed after 30 minutes.. |
The time course shows the efficient and fast binding of GFP to GFP-Trap. The high binding rate of the ChromoTek GFP-Trap enables a considerably shorter processing time than conventional anti-GFP antibodies. For GFP-Trap, a 30-60 min incubation time at 4°C is sufficient for a complete immunoprecipitation of GFP. Longer incubation times may increase the background of nonspecifically bound proteins instead.
GFP-Trap for a higher level of performance in various applications
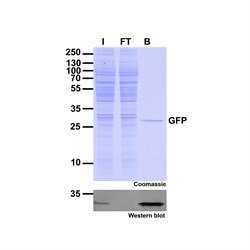 |
| Immunoprecipitation of GFP-fusion proteins: Just the protein of interest - no antibody contamination. I: Input, FT: Flow-Through, B: Bound. |
The GFP-Trap can be used for immunoprecipitation, including:
- IP of proteins expressed at low levels
- IP of proteins from large volumes, for example, secreted fusion proteins
- IP of membrane proteins in buffers containing detergents
- Co-IP, including Co-IP/MS with high reproducibility and low background
- On-bead enzymatic assays and on-bead digestion for mass spectrometry (MS) analysis
- Chromatin/RNA Immunoprecipitation (ChIP, RIP)
Stringent washing
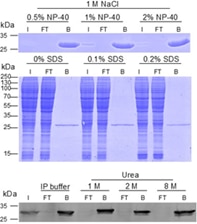 |
| Analysis of wash buffer compatibility: The GFP-Trap is compatible with common wash buffers and is also stable under harsh conditions. Even buffers containing 1 M NaCl and 2% NP-40, 1 M NaCl and 0.2% SDS, or 8 M Urea can be used for stringent washing without compromising GFP-Trap’s performance. |
The GFP-Trap is highly stable in contrast to conventional antibodies. Once bound to the GFP-fusion protein, very stringent washing conditions can be applied to remove unwanted proteins and reduce background. This stability also ensures that the GFP-Trap can be used in virtually any lysis buffer, e.g., in ubiquitination assays or in the presence of Urea, which is used for the total inactivation of any phosphatase activity in Co-IP/MS for phosphorylation studies. As you can see in the figure, GFP is always effectively bound, no GFP can be detected in flow-through as shown in the Western blot for up to 8 M Urea and in the SDS-PAGE for up to 2% NP-40 and 1 M NaCl.
Wash buffer compatibility
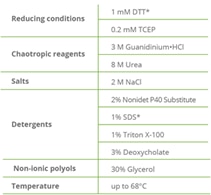 |
| GFP-Trap wash buffer compatibility. *GFP-Trap Magnetic Particles M-270: 10 mM DTT; 0.2% SDS. |
The wash buffer compatibility of the GFP-Trap bound to GFP has been tested under various conditions. Even denaturing conditions such as 8 M Urea have been shown not to interfere with the binding of GFP. We tested the binding of GFP-Trap to GFP under reducing conditions, with chaotropic reagents, different salt concentrations, non-ionic polyols, and under higher temperatures. The complex showed unique thermal and chemical stability.
Ready-to-use GFP-Trap formats
 |
| GFP-Trap Agarose: anti-GFP Nanobody conjugated to agarose beads |
The GFP-Trap consists of an anti-GFP Nanobody conjugated to different resins. The GFP-Trap is available as agarose beads, magnetic agarose beads, magnetic particles, or 96-well plates.
- GFP-Trap® Agarose for the lowest background and high binding capacity IP
- GFP-Trap® Magnetic Agarose for magnetic separation and high binding capacity IP
- GFP-Trap® Magnetic Particles M-270 for pull-down of large proteins/complexes
- GFP-Trap® Multiwell Plates for high-throughput applications and ELISA
- GFP-Trap kits that include lysis buffer for mammalian cells, wash, and elution buffers
For more details about the various matrices, please read our blog What GFP-Trap should I use for my immunoprecipitation?
IP of fusion proteins expressed at low levels
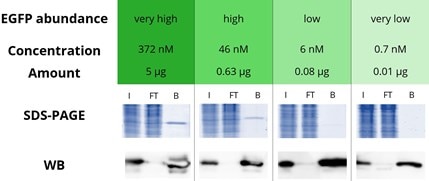 |
|
Immunoprecipitation of various concentrations of enhanced GFP (EGFP) with GFP-Trap Agarose: EGFP in a range from very high to very low amounts is effectively immunoprecipitated by the GFP-Trap: Very high corresponds to a concentration of 372 nM and an amount of 5 μg EGFP, The bound fraction in the Western blot (WB) shows a strong signal in all four concentrations whereas the flow-through is almost completely depleted. Especially for proteins expressed at very low levels, the strong band in the bound lane (WB) demonstrates the excellent properties of the GFP-Trap. Note that a shorter exposure time was used for the WB analysis of very high and high GFP concentrations. I: Input, FT: Flow-Through, B: Bound. |
The success of an IP depends on the expression level, specifically the concentration of the protein of interest (POI) in the sample: When the POI concentration equals the value of the dissociation constant of the affinity resin, 50% of the POI is bound (for more information see the white paper on steady state kinetics). Hence, a high affinity, i.e., low dissociation constant affinity resin is required for the effective IP of low expressed/low abundant POIs, POIs in large volumes such as cell supernatants, etc.
Because the concentration of POIs in the sample/lysis buffer is generally not known to researchers, we used GFP-Trap to immunoprecipitate EGFP samples at four different concentrations that are typical for very high, high, low, and very low expression/abundance levels when dissolved in standard lysis buffer volumes.
The effectiveness of the EGFP pull-down was analyzed by SDS-PAGE and Western blotting, indicating that the GFP-Trap was able to effectively immunoprecipitate EGFP at all concentration levels tested, including the very low abundance level because of the GFP-Trap’s extraordinarily high affinity with a dissociation constant KD of just 1 pM. In addition, no EGFP was detected in the flow-through fraction even when very high EGFP concentrations were applied, showing the broad applicability of the GFP-Trap.
 |
|
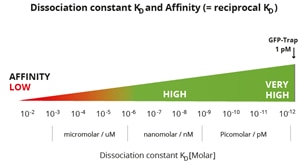
Classification of affinities from low to very high and their translation into dissociation constants. Note, GFP-Trap has a very high affinity with a KD of 10-12 M or 1 pM. Most conventional antibodies typically have dissociation constants from mid-nanomolar (10-7 M) to micromolar (10-5 M). ChromoTek’s Nano-Traps have higher affinities, i.e. a lower KD, which are in the single-digit nanomolar (10-9 M) to low picomolar (10-12 M) range and are highly suitable for IP of low abundance proteins. |
The GFP-Trap has an extraordinarily high affinity with a dissociation constant KD of just 1 pM and hence can effectively pull down low abundant GFP-fusion protein, which is present at low concentrations in the sample.
Co-IP for Mass Spectrometry (MS) analysis
 |
| Section of a mass spectrum |
The GFP-Trap is frequently used for Co-IP/MS assays because of its high reproducibility and low background, which are important for consistent results. Even in the presence of Urea, which can be used for instant inhibition of phosphorylation in the sample, the GFP-Trap maintains its high specificity and selectivity. After Co-IP, MS sample preparation does not require elution of the bound protein from the beads; instead, it can be conducted on bead either by following ChromoTek’s “on-bead digest protocol for mass spectrometry” or using the iST Kit for IP/Co-IP of GFP-fusion proteins & sample preparation MS. This convenient kit comprises the GFP-Trap and PreOmics iST buffers and cartridges required for easy, convenient, and effective proteomic sample preparation.
Immunoprecipitation of large proteins/protein complexes
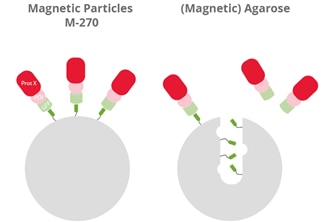 |
| Visualization of the binding of large GFP-fusion proteins (GFP, light green) + protein of interest (POI, pink) with an interacting partner (Prot X, red) to the GFP Nanobody (dark green) of GFP-Trap Magnetic Particles M-270 (left) and GFP-Trap (Magnetic) Agarose (right). |
GFP-Trap Magnetic Particles M-270 are recommended for the investigation of large proteins, protein complexes, or multimers (> 200 kDa). Magnetic Particles M-270 are solid beads, whereas Magnetic Agarose and Agarose are porous beads; large GFP fusion proteins, multimers, or bulky complexes with binding partners may be too large to diffuse into the pores of those agarose-based beads and, therefore, cannot be bound effectively. Please note, 200 kDa is an approximate value based on empirical studies. The cut-off can also depend on the protein shape.
Validation, characterization, and reliability
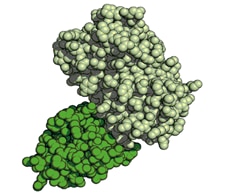 |
| Structure of GFP-GFP Nanobody complex. GFP (light green), GFP Nanobody (dark green). |
For robust and reproducible experiments, a thorough characterization of antibodies and Nanobodies is extraordinarily important. A comprehensive set of guidelines for the validation of antibodies was recently published in “A proposal for validation of antibodies” by the International Working Group for Antibody Validation (M. Uhlen et al. 2016). Based on this paper, ChromoTek Nanobodies are validated as follows:
- In the genetic approach, Nano-Traps are tested in their target application immunoprecipitation both on cell lines that express and do not express their cognate fluorescent protein or peptide tag.
- In addition, our Nanobodies are benchmarked with established conventional antibodies.
Both the sequence and structure of the GFP Nanobody used in the GFP-Trap are known. The recombinant production in combination with high QC standards ensures reliable and stable alpaca single domain antibody products with virtually no lot-to-lot variations.
Specificity of GFP-Trap
The GFP-Trap has a very high specificity and binds to GFP and the following derivatives:
AcGFP, Clover, eGFP, Emerald, GFP5, GFP Envy, GFP S65T, mGFP, mPhluorin, PA-GFP, Superfolder GFP, TagGFP, TagGFP2, monomeric eGFP A206K, CFP, YFP, Citrine, eCitrine, eYFP, Venus, Ypet, BFP
For the complete list, please click here: Fluorescent protein specificity table
For more details about GFP, read our blog GFP (green fluorescent protein): Properties, origin, specifications, tips.
What is the GFP-Trap? What is a Nanobody?
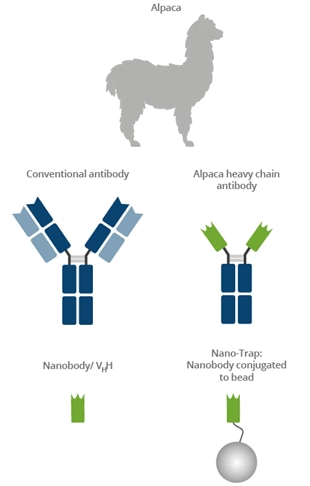 |
Camelids such as camels, llamas, and alpacas possess an immune repertoire of three subclass IgG antibodies: IgG1, IgG2, and IgG3. IgG1 is a conventional IgG composed of two heavy chains and two light chains. IgG2 and IgG3 are heavy-chain-only IgG antibodies (HCAbs) that can be distinguished by their hinge regions. These HCAbs lack the CH1 domain of the heavy chain and are devoid of any light chain. The binding domain of a heavy-chain-only IgG is called a Nanobody or VHH. Nanobodies have excellent binding properties and can be recombinantly expressed at constant high quality with no batch-to-batch variation. The GFP-Trap belongs to the ChromoTek Nano-Trap family. Nano-Traps are Nanobodies conjugated to beads and are ready-to-use. They enable superior IP performance.
Watch why GFP-Trap gives the best results for immunoprecipitation (IP)
Low background, no extra bands & high specificity will improve your pulldown assay significantly. Alpaca Alice shows how it works.
Related content:
What GFP-Trap should I use for my immunoprecipitation?
How to elute GFP-fusion protein from the GFP-Trap
How to conduct a Co-immunoprecipitation (Co-IP)
GFP (green fluorescent protein): Properties, origin, specifications, tips
Mass spec-compatible immunoprecipiation for GFP, mNeonGreen, Myc, RFP, Spot, and TurboGFP
The best anti-GFP antibody for immunoprecipitation: GFP-Trap
Green fluorescent protein (GFP) in plant research
New limit in protein complex stability: GFP-binding protein:GFP complex
Antibody Specificity and affinity in Immunoprecipitation
5 Tips for better immunoprecipitation
Request a Free Nano-Trap Sample
Nano-Trap system provides fast, reliable, and effective immunoprecipitation of fusion proteins. More than 3,000 peer-reviewed articles have already been published using ChromoTek's Nano-Traps.

