The “Heart” of Science
New challenges in cardiovascular research
Aging is an inevitable part of life and unfortunately poses the largest risk factor for cardiovascular disease.The heart undergoes continual remodelling in response to fluctuations in functional demand. Autophagy also plays a critical role in cell homeostasis, which is reflected in myocardial aging changes. There are several key genes that are involved in the regulation of cardiovascular health, including sirtuins, AMP-activated protein kinase, mammalian target of rapamycin, and insulin-like growth factor 1 (Figure 1).
Pathological hemodynamic overloading (e.g., hypertension and myocardial infarction) and unloading (e.g., prolonged bed rest and ventricular assist device) induce a broad spectrum of cardiovascular diseases (CVDs). In 2012, the WHO highlighted that more than 30% of the global population die because of CVDs (with the majority due to coronary heart disease and stroke). Smoking, chronic stress, high blood pressure, and diabetes are the most common factors for cardiovascular disease.
At present we have new capabilities and resources that can help to study the heart and its mechanism.
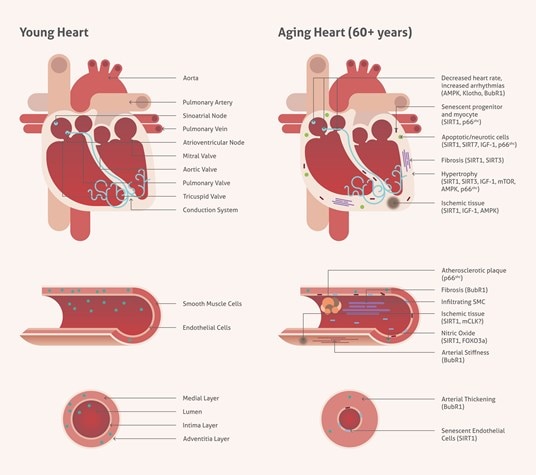
Figure 1. Age-associated changes in cardiovascular tissue.
New gene regions associated with high blood pressure
One of the latest discoveries relates to new genes associated with high blood pressure (hypertension) (1). Hypertension is a leading cause of heart disease, stroke, and death worldwide. The main factors contributing to hypertension are genetics and lifestyle factors such as diet, weight, alcohol consumption, and exercise. Scientists have found that high expression levels of 107 new gene regions in cardiovascular tissue or blood vessels can identify at-risk patients. Researchers tested over 9.8 million genetic variants from 420,000 UK Biobank participants and cross-referenced these with their blood pressure data. By using genetic tests, doctors can tailor proper treatment for individual patients.
iPSC-derived cardiomyocytes and their markers panel
Human iPSC-derived cardiomyocytes help scientists to build human cellular models and accelerate their research. The use of iPSC-derived cardiomyocytes as an in vitro research model has a major advantage over primary cells as they provide a continuous source of cardiomyocytes of the same genetic background for use in multiple sets of experiments. iPSC cardiomyocytes cells are a great tool for cardiotoxicity tests, drug screening, drug validation, and electrophysiology applications. Many in vitro iPSC-derived cardiomyocytes models display spontaneous cells beating and are fully defined by the cardiac-specific markers panel (such as cardiac troponin or ANKRD1). The measurement of Cardiac troponin I and Cardiac troponin T levels in serum is superior in regard to sensitivity and specificity to cardiac muscle enzyme measurements in the identification of heart muscle damage. Raised cardiac troponin concentrations are now accepted as the standard biochemical marker for the diagnosis of myocardial infarction (2).
Mature heart muscle cells from stem cells
Scientists are already able to generate mature and viable heart muscle from human or animal stem cells and implant them to newborn animals (3). Stem cells grown in lab dishes are not able to turn on genes needed for maturation, but growing them in a live animal enables the differentiation into cardiomyocytes. In this case, the hearts of host animals supply the biological signals and chemistry needed by the implanted immature heart muscle cells to progress and overcome the developmental blockade that traditionally stops their growth in laboratory conditions.
Advances in tissue engineering and heart regeneration
Since the heart cannot regenerate muscle tissue after a heart attack, part of the muscle wall is killed. The dead tissue can strain the surrounding muscle, leading to lethal heart enlargement. Researchers have grown heart tissue by seeding a mix of human cells onto a 1-micron-resolution scaffold made with a 3-D printer (4). The cells organized themselves in the scaffold to create engineered heart tissue that began beating within one day of seeding, and the speed and strength of contractions increased significantly over the next week (5). When the human-derived heart muscle patch was surgically placed into a mouse heart after a heart attack, it significantly improved heart function and decreased the amount of dead heart tissue.
New scientific discoveries will give us an ability to further improve the current state of the modeling of heart disease and heart repair. A future challenge will be to adapt these discoveries to advance new patient treatments.
References
- Genome-wide association analysis identifies novel blood pressure loci and offers biological insights into cardiovascular risk.
- Measurement of cardiac troponins.
- Neonatal Transplantation Confers Maturation of PSC-Derived Cardiomyocytes Conducive to Modeling Cardiomyopathy.
- From Microscale Devices to 3D Printing: Advances in Fabrication of 3D Cardiovascular Tissues.
- Myocardial Tissue Engineering With Cells Derived from Human Induced-Pluripotent Stem Cells and a Native-Like, High-Resolution, 3-Dimensionally Printed Scaffold.
