Dying to see what’s inside: Staining organelles
Visualizing the interior of organelles helps us to understand their molecular mechanisms and study cell abnormalities.
Firstly, what is an organelle? Organelles are small subcellular structures located in the cytoplasm of eukaryotic and prokaryotic cells, and in more complex eukaryotic cells, organelles are often enclosed by their own membrane. Each organelle performs a specialised function for that cell, much like an organ does for the body.
Beyond the use of organelle-specific protein markers, we can also use organelle-selective dyes to highlight structures of interest thus aiding your research into each of these specialist structures. Below we summarize how to get the most out of staining when identifying individual cell organelles (Figure 1).
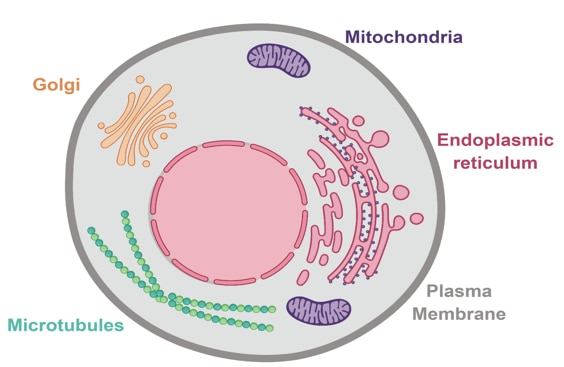
Figure 1. Animal cell highlighting some of the key organelles that can be stained with organelle-specific dyes.
Four considerations before selecting a dye:
Before we discuss the different dyes available for the different organelles, we must first consider certain points in order to ensure we get the best results from our stains.
1. Consider the membrane permeability of the dye.
DAPI has a low membrane permeability and is suitable for staining fixed cells. Hoechst (e.g., 33342) has a higher membrane permeability and can also be used in live cells.
2. Think about your fluorescent wavelengths.
For example, if you were identifying a protein of interest at the lipid membrane using a secondary antibody emitting light in the green channel, it is better to choose a lipid dye that emits light in the far-red channel. That way, you can avoid them interfering with each other.
3. Determining the concentration of your dye is crucial
Too low a concentration will not provide a strong visualization, while too high a concentration will be toxic to your samples.
4. Understand exactly how the dye works
Mitochondrial dyes like Rhodamine are dependent on membrane potential. They can only be applied to live cells, making them useful tools to analyze health and cell viability. Lysosome dyes require the acidic environment of this organelle to work properly. They also work best in live cells.
Different dyes for different organelles
1. Plasma membranes and the Golgi complex
The entire cell is enclosed by a phospholipid bilayer, the plasma membrane, and contains several membrane networks within the cytoplasm. The selective permeability of the plasma membrane and the formation of vesicles are essential for controlling the internal environment and the transport of proteins within the cell.
Importantly, please consider the membrane permeability of the dye. Membranes can be stained with lectins, which are carbohydrate-binding proteins that recognize and bind to certain parts of sugars.
Lectins such as wheat germ agglutinin (WGA) will stain the plasma membrane but also the Golgi, an organelle composed of many membrane sacs and vesicles involved in intracellular protein processing. Both structures are formed from the phospholipid bilayer, and both can be stained using lectins.
Please note: Some lectins are specific to particular glycolipids and glycoproteins. This is extremely useful when imaging multiple cell types. For example, lectin IsoB4 specifically stains endothelial cells by binding to their basal membrane.
2. ER and lipid droplets
The endoplasmic reticulum (ER) is another membrane system, continuous with the nuclear membrane and involved in the processing of lipids and proteins. The tubular cisternae and flattened sheets of the ER can make up about 10% of the entire cell volume. It can be classified into rough ER (associated with ribosomes and involved in protein production) or smooth ER (not associated with ribosomes and involved in lipid metabolism). Dyes such as Nile Red bind to lipid droplets and stain all lipophilic membranes, along with any intracellular lipid droplets. However, dyes such as this can show a lot of background staining, so optimize carefully to ensure reliable results.
Please note: Treating cells with oleic acid before staining with lipid-specific dyes will induce lipid droplet formation. This can be used as a positive control to check if the stain is working as expected.
3. Lysosomes
Lysosomes are described as the digestive system of the cell; they contain degradative enzymes. To maintain the function of these enzymes, the lysosome must remain in acidic conditions (around pH 5), in contrast to the neutral pH of the cytoplasm, meaning they require the active transport of protons from the cytoplasm. It is best to use a cell-permeable dye that specifically works in acidic environments and accumulates via the pH gradient into the lysosome.
Please note: Lysosome dyes such as Neutral Red require an acidic environment for this organelle and work best in live cells. One tip, is to first add the lysosome dye before subsequently fixing your cells, thus enabling the addition of protein-specific antibodies afterward.
4. Mitochondria
Mitochondria are considered to be the powerhouse of the cell. They produce almost 90% of the energy required for survival by the oxidative phosphorylation process, and are also a key player in apoptosis. They have two membranes that use a proton gradient to generate ATP, using it as intermediate energy storage. This membrane potential enables the use of mitochondrial dyes such as Rhodamine, a useful tool to analyze cell health and viability in live cells. However, it can only be applied right before fixation as the membrane potential is degraded.
Please note: Rhodamine is toxic and inhibits mitochondrial function.
5. Microtubules
Microtubules are one of the structural components of the cell that form the cytoskeleton, along with actin and intermediate filaments. While not strictly organelles, microtubules are important in understanding the fundamental processes governing cells and can also help when visualizing a whole cell. They can be identified using tubulin dyes, which, depending on the specific binding affinity, may affect the microtubule dynamics and could halt mitosis.
Please note: Use methanol fixation rather than paraformaldehyde. This will avoid interference with cross-linking proteins and will produce better-quality images.
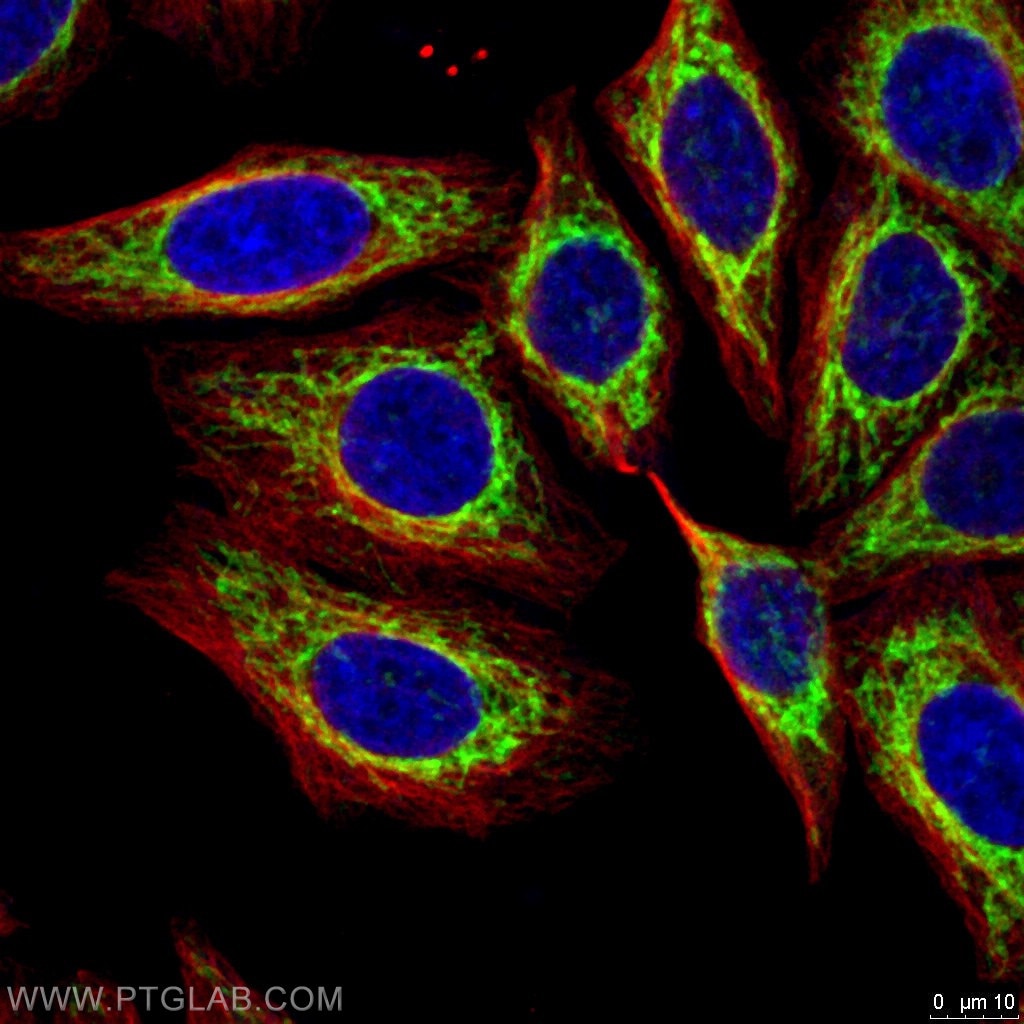
Figure 2. Immunofluorescent analysis of 4% PFA fixed HepG2 cells using FIS1 and Alpha Tubulin antibodies. Green: FIS1 (10956-1-AP); Red: Alpha Tubulin (66031-1-Ig)
Please remember to trust your dye!
Using dyes is a simple and effective way to visualize organelles in your research, alongside antibody staining. Ensure you thoroughly research the different options available to you, thus ensuring the best chances for success!
For more information on general staining tips, and multiple immunostaining technique check out technical tips on optimizing IF experiments.
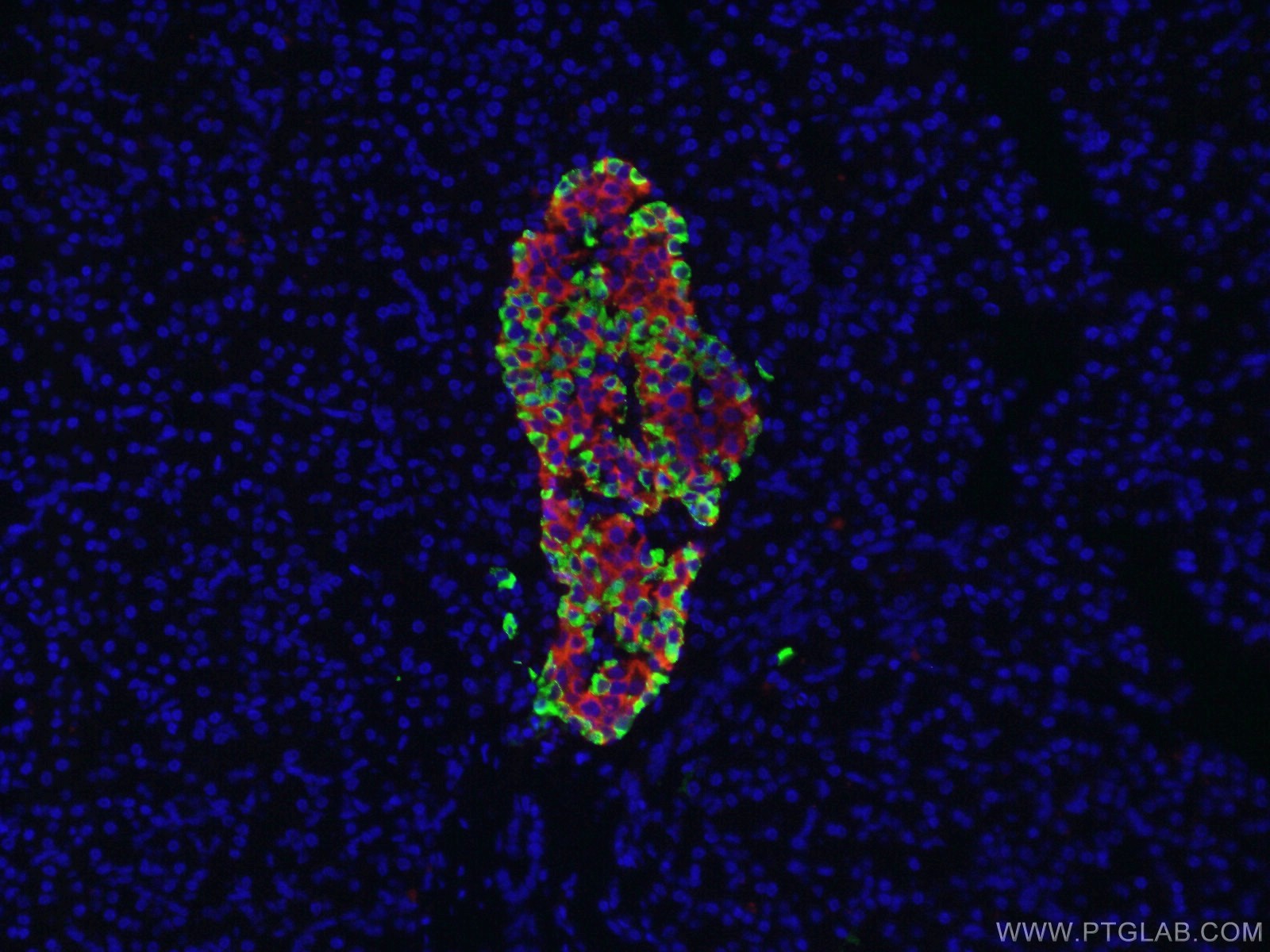
Figure 3. Immunofluorescent analysis of 4% PFA fixed human pancreas tissue using Glucagon and Insulin antibodies. Green: Glucagon (15954-1-AP); Red: Insulin (66198-1-Ig)