IF imaging: Widefield versus confocal microscopy
An overview of microscopy techniques and advice on when to use each method.
Introduction
Immunofluorescence (IF) is a technique used to visualize a protein of interest in its cellular context. It is based on staining cells with antibodies raised against a target protein that is directly conjugated with a fluorochrome or used together with fluorochrome-conjugated secondary antibodies.
Fluorescence microscopy is an imaging technique that uses fluorescent probes, dyes, or proteins (fluorophores) for sample labeling to generate an image. The image is based on the specific excitation of fluorescent molecules, where light is absorbed by a fluorophore and emitted at a longer wavelength.
Most fluorescence microscopes currently in use are epifluorescence widefield microscopes, where the excitation and detection of a signal are performed through the same light path (the objective). These microscopes are widely used in biology and are the basis for more advanced microscope designs, such as the confocal microscope (Figure 1).
Confocal and widefield microscopy
In a widefield microscope (Figure 1 A), fluorescence emitted by the labeled specimen is focused on the detector by the same objective that is used for the excitation light. The dichroic mirror acts as a wavelength-specific filter that transmits fluorescence to the eyepiece or a detector.
In a confocal microscope (Figure 1 B), light is emitted by a laser. A specific light wavelength passes through a pinhole and is reflected by the dichroic mirror toward the specimen. The pinhole allows only light from the plane of focus to reach the detector. This reduces the acquisition of out-of-focus light, thereby improving image quality (Figure 2). The excited fluorophore emits secondary fluorescence that passes through the dichroic mirror and is focused as a confocal point at the photomultiplier (PTM) detector pinhole.
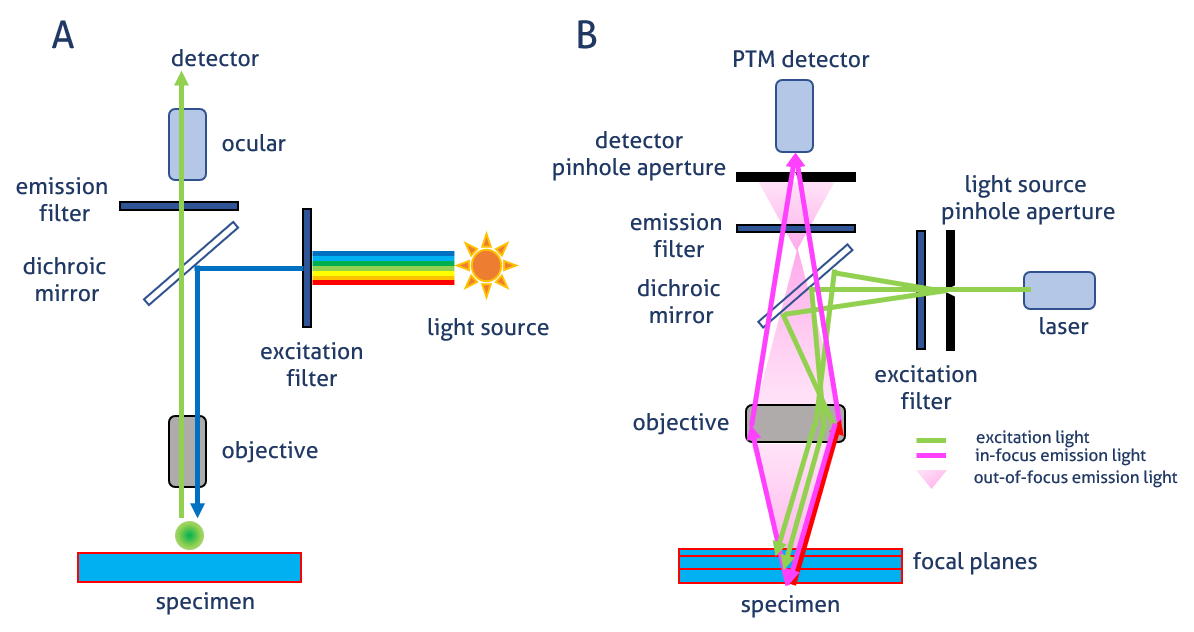
Figure 1. Image generation in widefield epifluorescence A) and confocal B) microscopes.
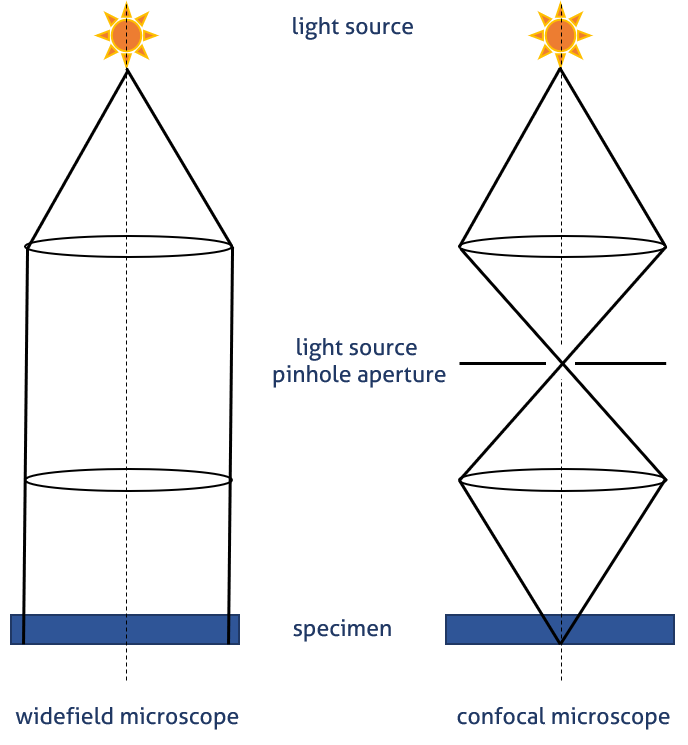
Figure 2. Light paths in widefield and confocal microscopy – visualization of the pinhole principle.
Confocal vs widefield microscopy: Advantages and disadvantages
A widefield microscope’s main advantages are that we get images quickly and can observe them directly in the ocular. The maintenance cost is low compared to the confocal microscope.
On the other hand, the widefield microscope carries a risk of high background. There is also the risk of channel-to-channel bleed-through (when fluorescent dyes have overlapping spectral profiles). Also, excitation wavelength bands depend on the filter sets available; this can be a limitation.
Using a confocal microscope, we can obtain a superior image quality and improve the signal-to-noise ratio. Due to light scattering, image blurring can be easily removed. A confocal’s flexibility in terms of excitation and emission of wavelength parameters reduces channel-to-channel bleed-through. Magnification can be adjusted electronically, and stitching across large surfaces of the specimen is also possible. A confocal microscope gives us a clear examination and 3D reconstruction of thick specimens due to effective Z-axis scanning.
However, it can be time-consuming to use a confocal microscope (depending on the scanning speed) and it has a more complicated image acquisition procedure compared to a widefield microscope. Also, confocal images are only obtained digitally from the PMT detector (the signal observed through the ocular lens is a widefield image).
Which microscope is best for my research?
For most uses, a widefield fluorescence microscope is sufficient and provides the best trade-off between quality, speed, ease of use, and cost. Therefore, it is a perfect tool for initial screens of protocols, and for live cell imaging applications where the speed of acquisition offers an advantage over scanning using confocal-based approaches (if the signal-to-noise ratio for a particular staining is sufficient).
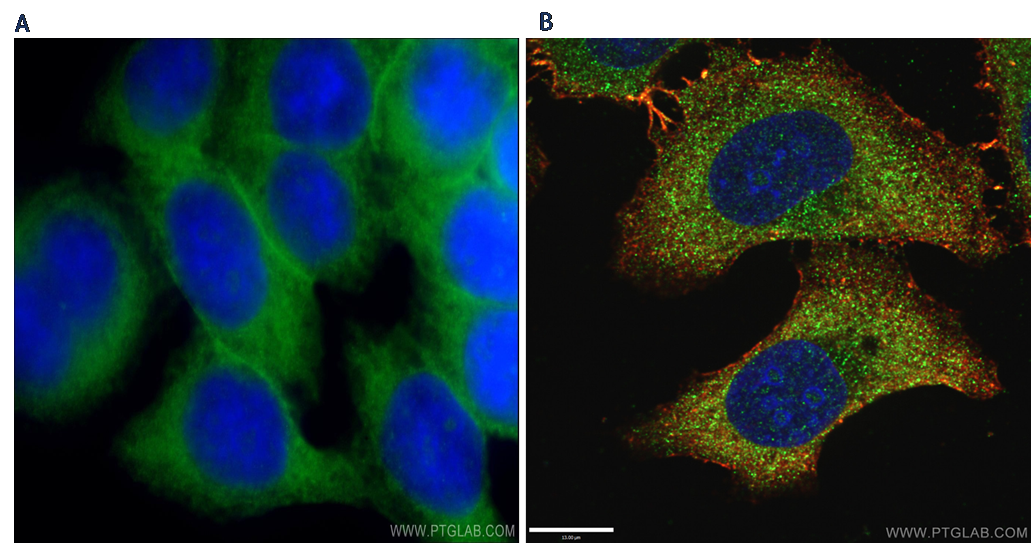
It is generally considered good practice to verify the quality of staining of your samples for confocal microscopy using a widefield microscope first (Figure 3). For example, it is advisable to use a confocal microscope for subcellular localization or protein–protein interaction studies, colocalizations, 3D imaging of thick tissues, or larger surfaces of a specimen (stitching between fields). It is also compatible with multi-fluorescence imaging, time-lapse imaging, FLIM, and FRAP measurements.
 |
|
Figure 3. A) Immunofluorescent analysis of HeLa cells using SLC2A1, GLUT1 antibody (21829- 1-AP) at a dilution of 1:50 and Alexa Fluor 488-conjugated AffiniPure Goat Anti-Rabbit IgG(H+L) by widefield microscope. B) Immunofluorescent analysis of (-20°C Ethanol) HepG2 cells fixed using LAMP1 antibody (55273-1-AP) at a dilution of 1:50 and Alexa Fluor 488-conjugated AffiniPure Goat Anti-Rabbit IgG(H+L)by confocal microscope.DAPI was used as the nuclear counterstain. |
Microscope setup & appropriate sample control panel
The setup of the microscope and appropriate sample controls are critical for IF imaging. Some important points to consider are listed below:
- Used fluorophores should have narrow spectral profiles of excitation and emission in order to reduce channel bleed-through when a multi-channel acquisition is planned.
- Controls and samples should be imaged side by side using identical settings to eliminate the background and false positive signal.
- You need to include the following set of experimental controls in each experiment:
- knock-out cells or tissue/cell line, positive and negative control
- sample stained only with secondary antibody
- in case of multiple staining, each antibody should be tested individually.
The microscope you choose depends on the nature of the individual experiment and the questions you wish to answer in the most efficient manner.
Related Content
Multiplexing with Same-Species Antibodies for Immunofluorescence
CoraLite conjugated antibodies
Advantages of CoraLite direct staining for IF
Direct vs indirect immunofluorescence
How to reduce autofluorescence
Save time on your immunofluorescence experiments
9 Tips to optimize your IF experiments
Conjugation of fluorescent dyes to Nanobodies
GFP Nanobody for better images in immunofluorescence
GFP and RFP-Booster for better immunofluorescence imaging
Support
Newsletter Signup
Stay up-to-date with our latest news and events. New to Proteintech? Get 10% off your first order when you sign up.
