GFP-Booster: GFP Nanobody for better images in immunofluorescence
Higher resolution and better tissue penetration
Since the discovery of the green fluorescent protein (GFP) it has been widely applied in fluorescence microscopy as a tool to study proteins in their native cellular environment. However, GFP has several intrinsic limitations, such as low signal intensity, fast photo bleaching and signal loss after chemical treatment. The GFP-Booster, which is composed of an anti-GFP Nanobody conjugated to a fluorescent dye, enhances, stabilizes, and re-establishes the signal of GFP fusion proteins in immunofluorescence. In this blog, we answer why the small size of the GFP-Booster is of advantage compared to regular antibodies in immunofluorescence and provide an overview of the GFP variants bound by the GFP-Booster as well as the available dyes.
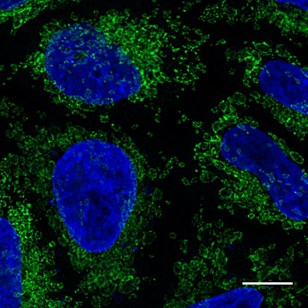
HeLa cells transiently transfected with Tom70-eGFP and immunostained with GFP-Booster Alexa Fluor® 488 (green). Nuclei were stained with DAPI (blue). Scale bar, 10 µm. Images were recorded at the Core Facility Bioimaging at the Biomedical Center, LMU Munich.
- What are the limitations of GFP in fluorescence microscopy?
- What is a GFP-Booster and how can it be applied?
- Size matters: Why is the small size of the GFP-Booster of advantage?
- Why is the GFP-Booster superior for tissue penetration?
- Why does the GFP-Booster minimize the epitope-label displacement?
- Which fluorescent proteins can be bound by the GFP-Booster?
- Which fluorescent dyes are available?
- How was the GFP-Booster validated?
GFP is an excellent tool to study proteins and has had a major impact on scientific research. “The discovery and development of the green fluorescent protein, GFP” was awarded the Nobel Prize in Chemistry in 2008 and enabled many new scientific discoveries. GFP, often fused to a protein of interest (POI), can be used to study protein expression, protein localization, and protein dynamics in living cells and organisms.
However, fluorescent proteins such as GFP also have several limitations:
-
- The signal intensities of fixed samples from cells that express GFP fusion proteins at physiological expression levels are usually very low and, therefore, difficult to detect.
- GFP is prone to bleaching. Neither the photostability nor quantum efficiency of GFP and its derivatives are generally sufficient for super-resolution microscopy.
- Many cell biological methods such as EdU-Click-iT™ treatment, heat denaturation for FiSH, or other harsh treatments can denature GFP and therefore lead to loss of the fluorescent signal.
The GFP-Booster can help to overcome these limitations, as it reactivates, stabilizes, and enhances the fluorescent signal of GFP fusion proteins.
The GFP-Booster comprises a small anti-GFP antibody fragment conjugated to a fluorescent dye. The anti-GFP antibody fragment is called an anti-GFP Nanobody or anti-GFP VHH. Nanobodies are very small antibody fragments. They are derived from so-called heavy-chain-only IgG antibodies, which can be found in alpacas, camels, and llamas. These heavy-chain-only IgG antibodies lack a light chain and, therefore, bind to the epitope with a single protein-binding domain. The GFP-Booster is monovalent and offered with various Alexa Fluor® or ATTO dyes. When it binds to a GFP-tagged protein, the signal of the conjugated fluorescent dye can be detected in immunofluorescence applications such as epi-fluorescence, confocal, and super-resolution microscopy.
Nanobody (dark green), fluorescent dyes (red), and antibody Fc-domains (blue).
A regular antibody consists of two heavy (~50 kDa) and two light (~25 kDa) chains, summing up to roughly 150 kDa. Often complexes consisting of a primary and several secondary antibodies are used in immunofluorescence, increasing the size to over 300 kDa. In contrast, the binding domain of a Nanobody is only ~15 kDa in size, which provides several advantages in immunofluorescence, such as better tissue penetration or less epitope-label displacement.
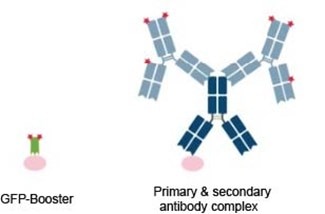
Schematic comparison of GFP-Booster (green) vs. a conventional primary antibody (dark blue) and secondary antibodies (light blue) conjugated to fluorescent dyes (red). The epitope is shown in pink.
The GFP-Booster is about 10 times smaller than a conventional IgG antibody. In crowded cellular environments and tissues, its small size enables better and faster tissue penetration. Especially when staining organs or whole animals, the penetration rate directly influences image quality and incubation time.
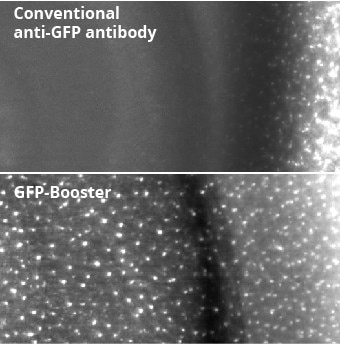
The smaller the better – Nanobodies, e.g., Nano-Boosters, penetrate tissue better than conventional antibodies: The comparison of conventional anti-GFP antibody and GFP-Booster shows the superior tissue penetration rate of GFP-Booster. Fluorescent images of transgenic mouse tissue expressing Cx3Cr1-EGFP. EGFP signal was enhanced with conventional anti-GFP antibody (top image) or with GFP-Booster (bottom image).
Especially in super-resolution microscopy the distance between the fluorophore and the epitope can be an important factor to consider. The small size of the GFP-Booster minimizes the epitope-label displacement and therefore the localization error. With the GFP-Booster, the distance between the fluorophore and the epitope is about 2 nm, compared to about 25 nm with a conventional primary and secondary antibody complex.
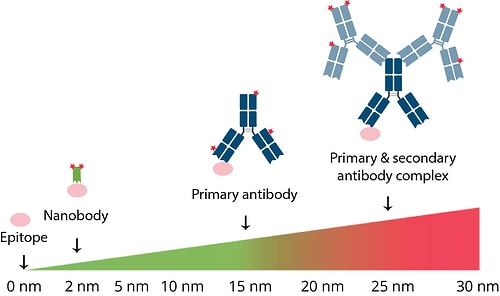
Distance between the epitope and fluorophore with a labeled Nanobody (green), a labeled primary IgG antibody (dark blue), and a complex of primary IgG antibody (dark blue) and labeled secondary IgG antibodies (light blue). Fluorescent dyes are shown in red; epitope is shown in pink.
The GFP-Booster binds not only to GFP but also to many GFP derivatives:
-
- CFP
- AcGFP, EGFP, GFP, GFP S65T, mClover, Monomeric EGFP A206K, pHluorin, PA-GFP, Superfolder GFP, TagGFP, TagGFP2
- Citrine, Ecitrine, EYFP, Venus, YFP, Ypet
It does not bind to mNeonGreen, TurboGFP, RFP, or mCherry.
For increased flexibility in experiments, the GFP-Booster is available conjugated to different fluorescent dyes:
These provide
-
- strong signal for confocal and standard microscopy.
- different options for super-resolution microscopy including STED, STORM, MINFLUX.
- constant degree of labeling for higher resolution.
For specific applications, special dyes can be self-conjugated to the unconjugated GFP-Booster, i.e., ChromoTek’s unconjugated GFP VHH/ Nanobody. You can find more detailed information about the general labeling chemistries of Nanobodies in our blog Conjugation of fluorescent dyes to Nanobodies.
The GFP-Booster is based on a monoclonal Nanobody that is characterized and tested in-depth. It is validated by a genetic approach, meaning it is tested for immunofluorescence on cell lines that express GFP as well as on control lines lacking GFP. The GFP-Booster is recombinantly manufactured and every lot is tested according to strict QC guidelines. This means there is a very high batch-to-batch consistency, which is important for reproducible results.
Related Content
Why are recombinant Nanobodies/ VHHs beneficial?
Conjugation of fluorescent dyes to Nanobodies
GFP and RFP-Booster for better immunofluorescence imaging
New anti-mNeonGreen antibody & Nano-Trap for Immunofluorescence & Immunoprecipitation
The advantages of CoraLite direct staining for IF
Advantages of recombinant Nano-Secondaries

Support
Newsletter Signup
Stay up-to-date with our latest news and events. New to Proteintech? Get 10% off your first order when you sign up.
