What is the difference between necrosis and apoptosis?
Understand the differences between regulated and unregulated cell death.
Cell death is a naturally occurring phenomenon in multicellular organisms and cells can die as a result of internal and external stimuli. Cell death can be broadly classified into regulated cell death (RCD) and unregulated cell death a.k.a. accidental cell death (ACD). While ACD is a disordered process resulting from an unexpected attack or injury, RCD proceeds through precise signaling pathways which result in defined biochemical and functional outcomes. Apoptosis is the earliest discovered and the most extensively studied RCD pathway. Several non-apoptotic RCD pathways including pyroptosis, ferroptosis and necroptosis have gained significant attention in recent years due to their potential in cancer therapy and their involvement in the pathogenesis of multiple diseases. Here we will discuss the key features of apoptosis and the most common form of ACD termed necrosis while highlighting the key difference between the two cell death pathways, and taking a closer look at necroptosis, a regulated form of necrosis.
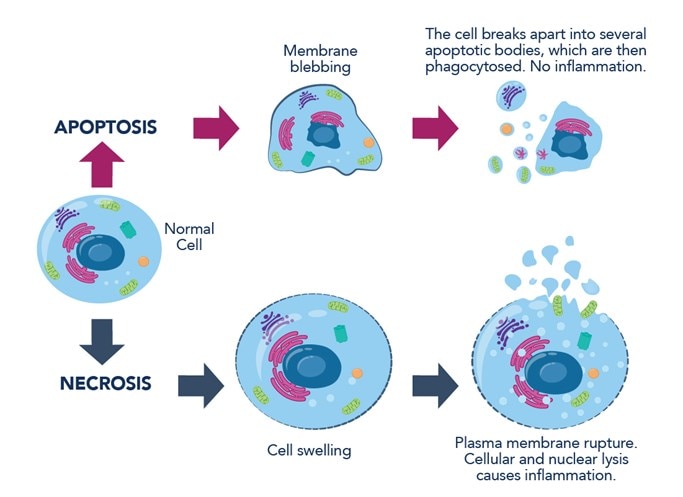
Figure 1: Hallmarks of apoptosis and necrosis.
What is Apoptosis?
Apoptosis is a classical form of RCD pathway that plays a critical role in both normal physiological as well as pathological processes. It can be triggered via various physical, chemical, and biological factors, and its cellular response is tightly regulated. The controlled degradation of cellular components that occurs during apoptosis is regulated by caspases, which are a family of proteases activated during apoptosis (more details here: Caspase family).
In healthy cells, caspases exist as proenzymes in their inactive forms. Apoptotic signaling activates a caspase cascade (caspase -2,-8, -9, and -10, called initiator caspases). Next, the initiator caspases in turn cleave and activate downstream effector caspases (caspase -3, -6, and -7). Effector caspases execute apoptosis by cleaving targeted cellular proteins. Stimuli initiating apoptosis can be internal (e.g., DNA damage, ER stress, increased ROS levels, cell defects during mitosis), or external, whereby extracellular stimuli are detected by cells via plasma membrane receptors.
Extrinsic apoptosis
There are two main receptor types for externally induced apoptosis. Death receptors (e.g., TNF, Fas receptors) bind their extracellular ligands (TNF-alpha, FasL – Fas ligand, respectively). This prompts their activation and assembly into complexes, leading to the activation of intracellular caspases. The other apoptotic receptors are called dependence receptors (e.g., DCC and PTCH1), which work on a completely opposite sensing mechanism. In physiological conditions, they respond to trophic factors and act as an anti-apoptotic stimulus. However, when their ligand falls below a certain level in the extracellular space, ligand-free receptors trigger the apoptotic response.
Intrinsic apoptosis
Intrinsic apoptosis is mediated by mitochondria-associated BCL-2 family proteins – BAX and BAK (BAX antibody: 50599-2-Ig). BAK is a transmembrane protein of the outer mitochondrial membrane. Upon apoptosis induction, BAX undergoes conformational change. This exposes its transmembrane domain, leading to the insertion of BAX into the outer mitochondrial membrane. BAX-BAK heterodimers form mitochondrial pores, which leads to the release of mitochondrial proteins into the cytoplasm, including cytochrome c and DIABLO/Smac proteins, that can activate caspases.
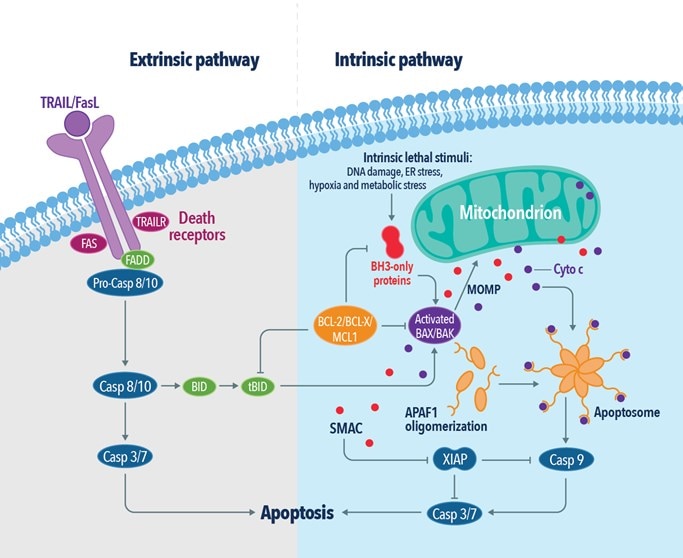
Figure 2: The extrinsic and intrinsic pathways of apoptosis.
Changes during apoptosis
Apoptosis is characterized by a series highly regulated biochemical events that lead to significant changes in cellular morphology and ultimately cell death (Table 1). In the earlier phases, a cell undergoing apoptosis loses cell contacts and changes shape. Chromatin condenses in the nucleus and moves toward the nuclear envelope. Condensation of the nucleus (pyknosis) initiates DNA degradation. Loss of water results in significant cell shrinkage and blebbing of the plasma membrane with little or no morphological changes to the other cellular organelles. Phosphatidylserine, a lipid present only in the inner layer of the plasma membrane, is now also visible in the outer layer. Nucleus and cytoplasm fragment into apoptotic bodies. Released cellular proteases lead to disintegration of the cellular skeleton, membranes, and proteins. Neighboring macrophages recognize, engulf, and digest apoptotic bodies, completing the process.
 |
 |
|
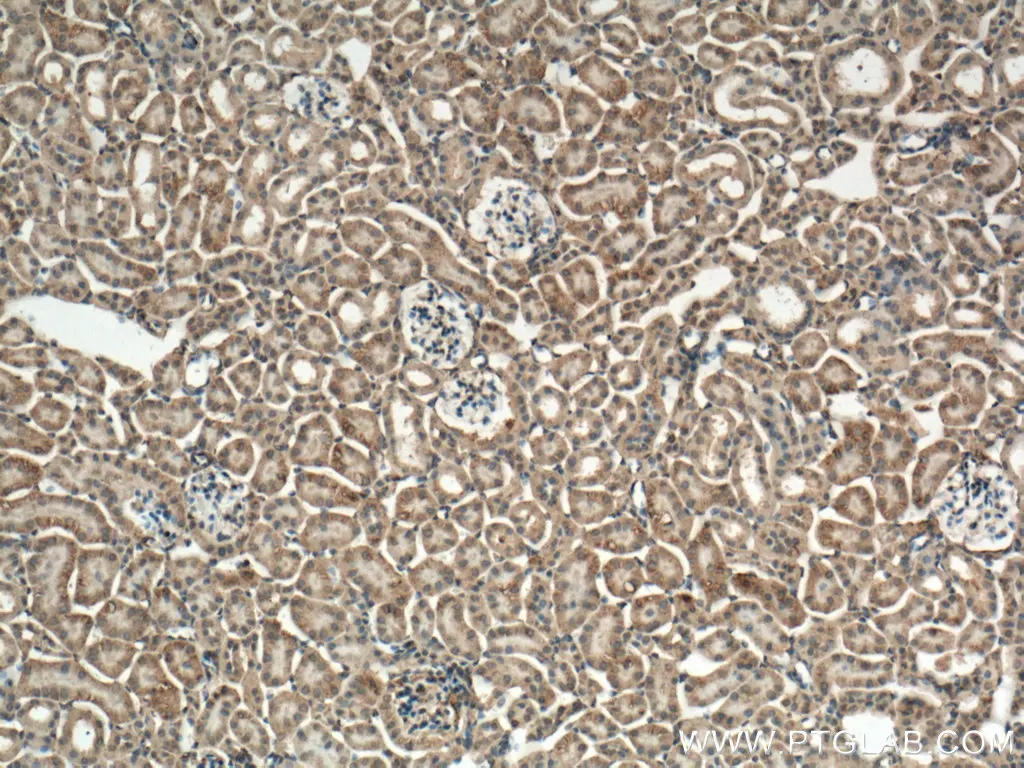
Figure 3: IHC analysis of mouse kidney tissue using Caspase- 3 antibody (66470-2-Ig) at dilution of 1:300 (under 10x lens). |
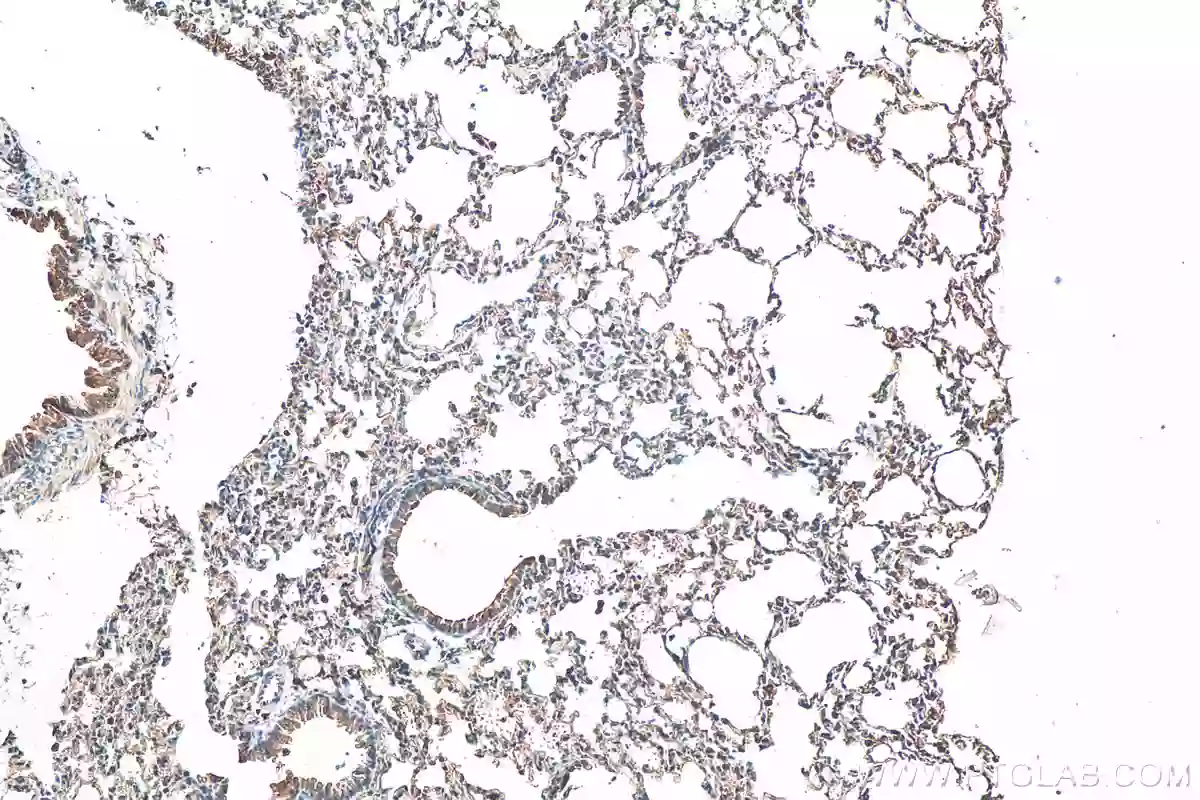
Figure 4: IHC analysis of mouse lung tissue slide using BAX antibody (50599-2-Ig) at dilution of 1:2000 (under 10x lens). |
What is necrosis?
Necrosis is a form of ACD resulting from internal or external stresses such as mechanistic injuries, chemical agents, or pathogens. The process is usually rapid and leads to cell swelling (oncosis) and bursting due to loss of osmotic pressure (Table 1).
Changes during necrosis
During necrosis, the loss of plasma membrane integrity induces cellular contents to escape to the extracellular space, causing inflammatory responses. Cell disintegration is preceded by a series of morphological changes, including disruption of cell organelles, such as swelling of the ER and mitochondria, or decay of the Golgi apparatus. An influx of calcium ions from the extracellular matrix activates intracellular nucleases that fragment DNA. Freed lysosomal hydrolases contribute to the degradation of nucleic acids and proteins. Decay products activate leukocytes, lymphocytes, and macrophages that phagocytose the remnants of dead cells.
Necroptosis – regulated necrosis
While necrosis was considered to be strictly an unregulated form of cell death previously, recent studies have identified a regulated form of cell death with necrotic phenotype, termed necroptosis. It arises in response to various stress stimuli including interferons, death ligands, or Toll-like receptors. Necroptosis is highly immunogenic in nature and is often utilized by host cells as a defense mechanism against pathogens. Moreover, since the necroptotic pathway does not rely on caspase activation, it is activated as an alternative form of RCD when the apoptotic pathway is inhibited by certain factors such as viral proteins or genetic defects.
Of the above-mentioned factors, necroptosis mediated by TNFα and its receptor TNFR1 is the most well-characterized pathway. In this pathway, binding of TNFα to TNFR leads to the assembly of a multimeric protein complex consisting of TRADD, TRAF2/5, cIAP, and RIP1. RIP1 plays a critical role in determining cell fate by committing cells to either cell survival or cell death pathways. While polyubiquitination of RIP1 by cIAP1/2 activates the NF-κB cell survival pathway, inhibition of RIP1 ubiquitination leads to the activation of either apoptotic or necroptotic cell death pathways. The association of RIP1 with FADD and procaspase-8 results in apoptotic cell death by caspase-8 activation and subsequent RIP1 cleavage. In the absence of caspase-8 activation, RIP1 associates with RIP3 to form the necrosome. The necrosome then phosphorylates MLKL leading to its oligomerization and subsequent insertion into the plasma membrane. Membrane insertion of MLKL results in cell rupture and the release of inflammatory molecules such as DAMPs into the extracellular space.
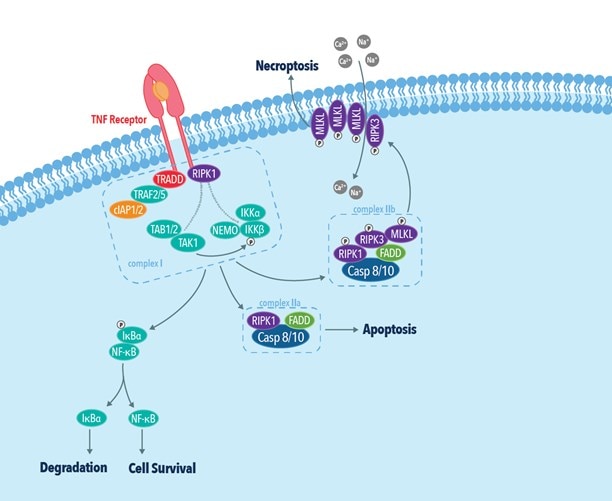
Figure 5: The TNF receptor dependent necroptotic pathway.
 |
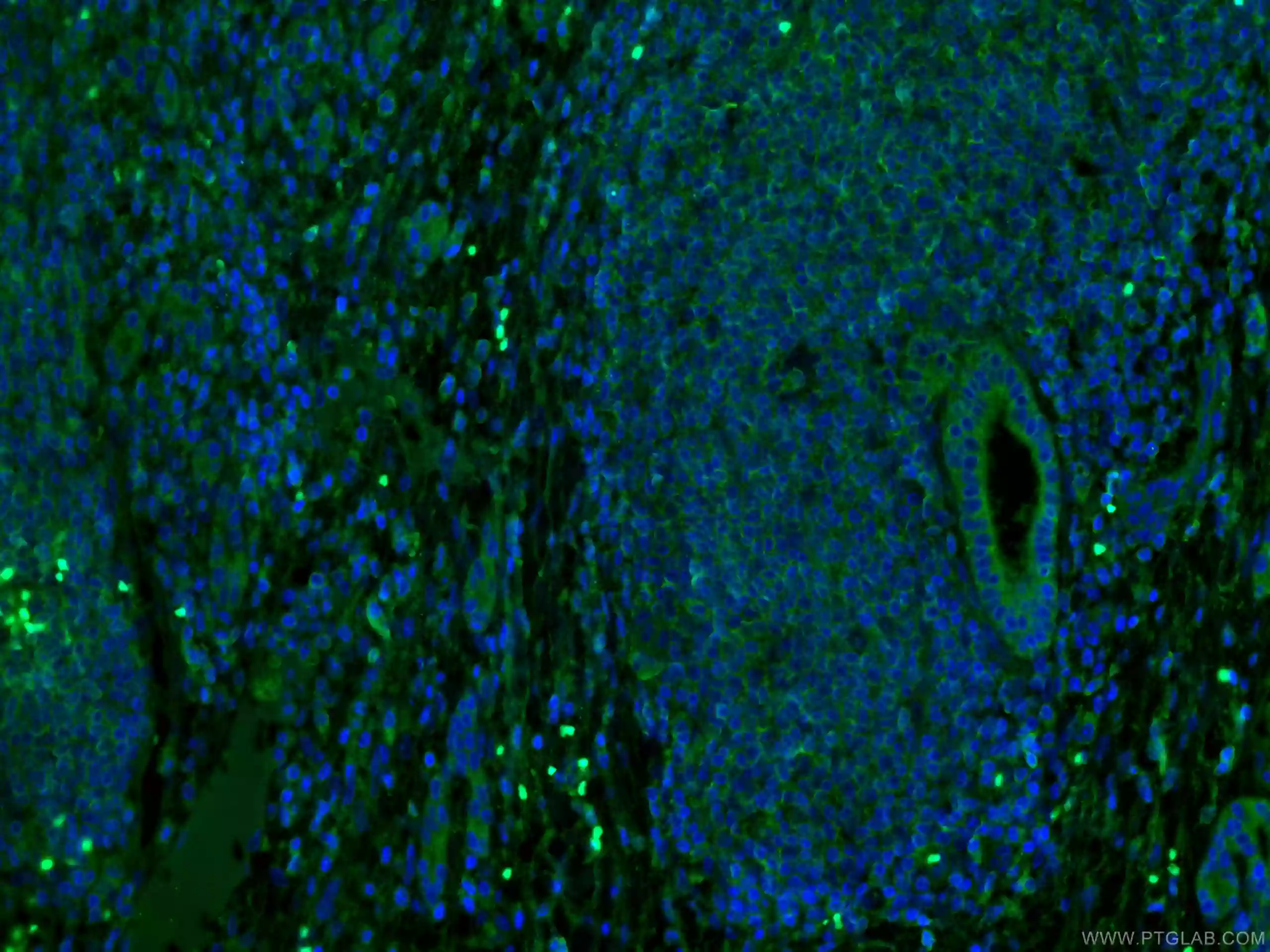 |
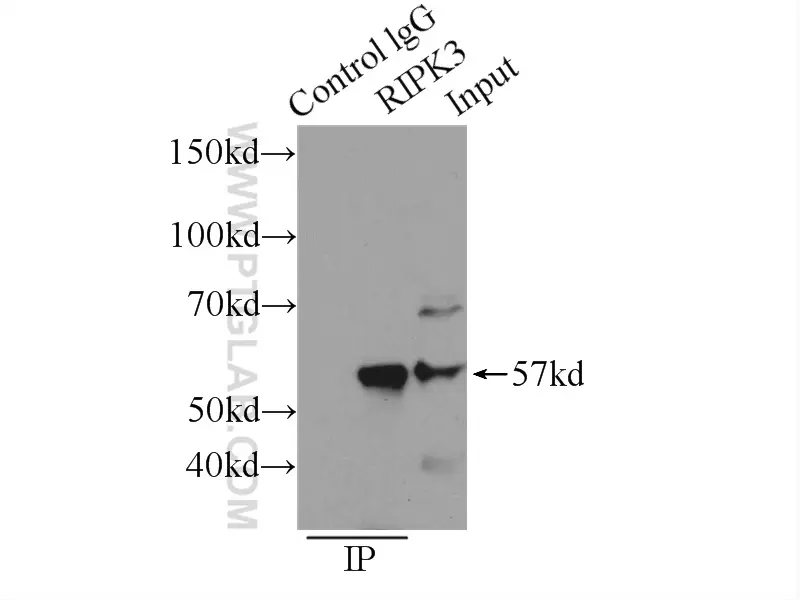
| Figure 6: IP analysis of RIP3 (IP:17563-1-AP, 3ug; Detection:17563-1-AP 1:300) in SW 1990 cell lysate. | Figure 7: IF analysis of human liver cancer tissue using MLKL antibody (66675-1-Ig) at dilution of 1:100 and CoraLite488-Conjugated AffiniPure Goat Anti-Mouse IgG(H+L). |
Table 1. Physiological events during apoptosis and necrosis.
|
|
Apoptosis |
Necrosis |
|
Morphological changes |
Cell |
Shrinkage and loss of cell-cell contacts; apoptotic cells phagocytose neighboring cells |
Swelling; cell lysis |
|
Plasma membrane |
Blebbing with intact cell integrity, formation of apoptotic bodies at late stages |
Loss of integrity; increased permeability |
|
|
Organelles |
No visible changes |
Cell fragmentation |
|
|
Nucleus |
Chromatin condensation, fragmentation |
Condensation of chromatin and disintegration of the nucleus |
|
|
Mitochondrion |
Decrease in membrane potential, swelling |
Non-functional, swelling and fragmentation |
|
Biochemical changes |
DNA |
Endonuclease-induced cleavage to fragments of specific lengths (DNA laddering) |
Random degradation of genomic DNA |
|
Proteins |
Kinases activation; phosphatases, caspases, and nucleases |
Unspecific degradation |
|
|
Anti-apoptotic proteins |
Bcl-2 family proteins, Inhibitor of Apoptosis Proteins (IAPs), caspase inhibitors |
Bcl-2 expression in some cases |
|
|
Energy |
ATP-dependent |
ATP-independent |
|
Tissue response |
|
|
|
Post-death clearance |
Phagocytosis |
Cell lysis |
|
Final remarks
Apoptosis is a form of programmed cell death that can be initiated by a number of internal and external routes; it is a well-controlled process that results in the slow turnover of cell remnants and phagocytosis by neighboring macrophages. In contrast, necrosis is caused by external factors that lead to irreversible cell injury, with loss of plasma membrane integrity and rapid death often resulting in activation of the immune system.
Apoptosis is a critical component of several normal physiological processes including embryonic development, tissue homeostasis, immune response, and aging and is therefore non-inflammatory in nature. Dysregulation of the apoptotic pathway however has been associated with multiple pathological conditions including neurodegenerative diseases, ischemia, cancer, and autoimmune disorders. Necrosis, on the other hand is typically not seen under normal physiological conditions but is triggered in response to extreme physical or chemical cellular injury leading to inflammation. Inhibition of apoptotis by certain factors leads to the activation of necroptosis, a regulated form of necrotic cell death.
References
- Molecular mechanisms of cell death: recommendations of the Nomenclature Committee on Cell Death 2018.
- Apoptosis: A Review of Programmed Cell Death.
- Cell death: apoptosis versus necrosis (review).
- Receptor interacting protein kinase-3 determines cellular necrotic response to TNF-alpha.
- Phosphorylation-driven assembly of the RIP1-RIP3 complex regulates programmed necrosis and virus-induced inflammation.
- RIP3, an energy metabolism regulator that switches TNF-induced cell death from apoptosis to necrosis.
- RIP kinases as modulators of inflammation and immunity.
Related Content
Metabolic regulation of cell death
Caspase cascade antibodies: Caspases play an essential role in apoptosis
How do I know if the antibody will cross-react?
Protein or peptide antigen? Advantages and disadvantages
Want to upgrade your immunofluorescence workflow? Go Direct!
Why are recombinant Nanobodies/ VHHs beneficial?
How to write a good scientific abstract?
Learn how to save precious hours on your IP, IF, and western blotting experiments
Support
Newsletter Signup
Stay up-to-date with our latest news and events. New to Proteintech? Get 10% off your first order when you sign up.

